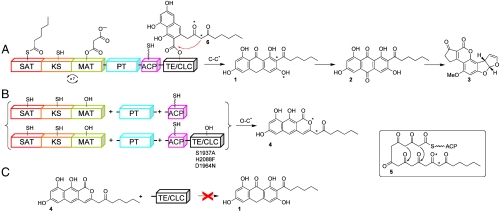
Fig. 1.
PksA-catalyzed biosynthesis of norsolorinic acid anthrone (noranthrone, 1), the polyketide precursor of aflatoxin B1 (3). The domain architecture for PksA is shown, which includes SAT, β-ketoacyl synthase, MAT, PT, ACP, and TE/CLC domains. (A) The SAT domain receives a C6-fatty acid starter unit from an associated fungal FAS (HexA/HexB, not shown) and transfers it onto the ACP for chain elongation. The KS accepts the hexanoyl-ACP unit and subsequent malonate extender units are loaded onto the ACP from the MAT domain for chain extension to generate the linear poly-β-keto ACP-bound intermediate 5. The linear intermediate is then cyclized and aromatized in the PT domain. The resulting bicyclic intermediate is ultimately transferred from the ACP to the TE/CLC domain (intermediate 6) and undergoes Claisen-type C–C bond cyclization to release the product norsolorinic acid anthrone (noranthrone, 1), which spontaneously oxidizes in vitro to norsolorinic acid (2). (B) Three-part multidomain combination strategy. Product derailment (O–C cyclization, 4) primarily occurs without a competent TE/CLC domain (catalytic triad mutations S1937A, H2088F, and D1964N). (C) TE/CLC must accept a bicyclic ACP-bound intermediate to yield 6. The norpyrone product 4 is not a substrate for the TE/CLC domain.