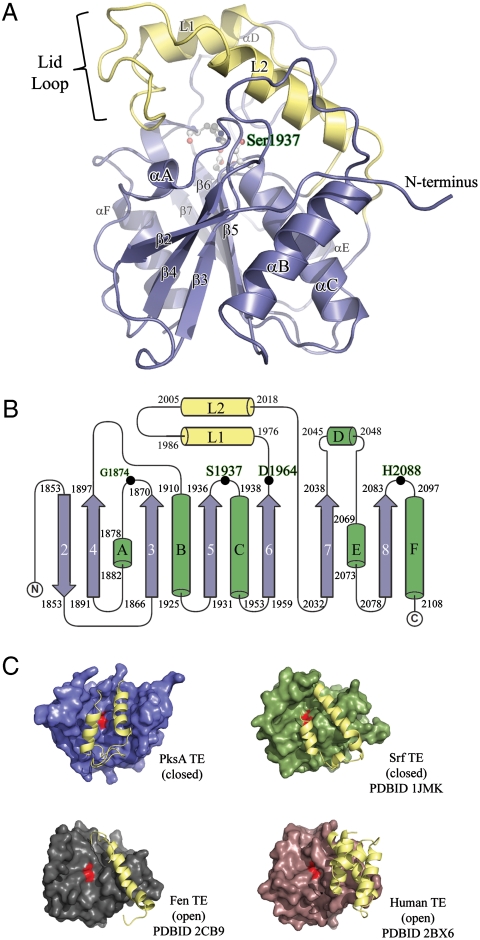
Fig. 3.
Overall structure of the PksA TE domain. (A) Ribbon diagram of the TE α/β-hydrolase fold with the core domain shown in blue and the unique lid in yellow. The location of the S1937-H2088-D1964 catalytic triad and the oxyanion site residue G1874 is shown in balls-and-sticks and labeled green. The position of the lid-loop, which presumably opens upon presentation of substrate by ACP, is closed. (B) Topology diagram of PksA TE showing the location of the catalytic triad residues along with G1874 (Black Dots), which is thought to act as the oxyanion hole for the reaction, and the lid region (Yellow) as an insertion between β6 and β7, a common feature of FAS, PKS and NRPS TE domains. The core sheets and helices are colored blue and green, respectively, and are numbered as the canonical α/β-hydrolase fold showing that PksA TE lacks the first N-terminal sheet necessary for dimerization. (C) Comparison of open and closed lid conformations found in TE crystal structures.