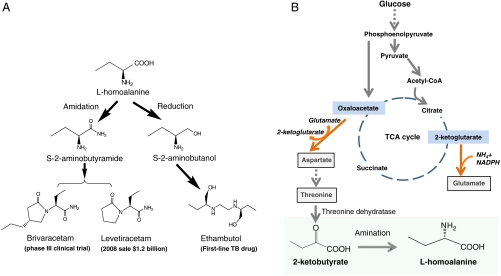
Fig. 1.
Biosynthesis of L-homoalanine and its pharmaceutical applications. (A) Chemical synthetic routes of antiepileptic and antituberculosis drugs from the chiral intermediate L-homoalanine. (B) Constructing a nonnatural metabolic pathway for L-homoalanine fermentation. Engineered E. coli can overproduce the natural amino acid threonine from glucose. Threonine is converted to 2-ketobutyrate by threonine dehydratase. Then L-homoalanine is synthesized from 2-ketobutyrate by amination.