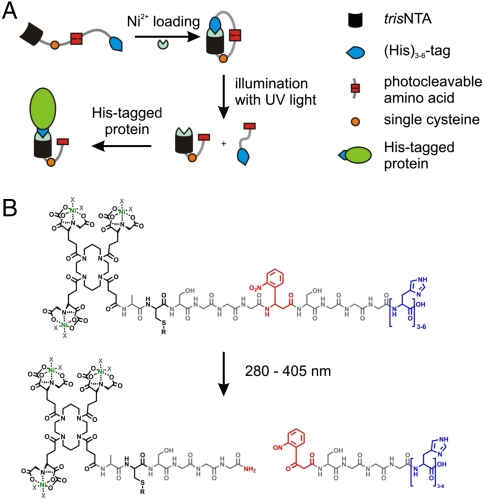
Fig. 1.
Design of the photoactivatable tris-NTAs (PA tris-NTAs). (A) Schematic illustration of the PA tris-NTA concept relying on a tris-NTA moiety, a single cysteine for further modifications, the UV-sensitive amino acid (Anp), and cumulated histidines (blue). Addition of Ni2+ ions forces PA tris-NTA in a closed self-inactivating conformation. Photocleavage disrupts the intramolecular self-inactivation, allowing subsequent binding to His-tagged proteins. (B) Chemical structure of the PA tris-NTA compounds. Upon illumination the peptide backbone is photocleaved at the Anp (red).