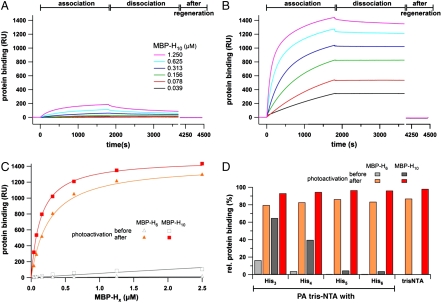
Fig. 3.
Light-induced interaction of His-tagged proteins followed by SPR. The association and dissociation kinetics of MBP-H10 before (A) and after photoactivation (B) reveal a drastic change in affinity. Before photoactivation almost no interaction is observed, while after illumination His-tagged proteins are stably bound at high density. (C) Protein binding to the PA tris-NTA-His5 surface (30 min after dissociation) are plotted against the concentration of MBP-H6 and MBP-H10 before (open) and after (closed symbols) photoactivation. (D) Comparison of the relative binding signals obtained for MBP-H6 and MBP-H10 (625 nM each) interacting with different PA tris-NTAs. The relative amounts of bound proteins (30 min after dissociation) are shown before (gray) and after photoactivation (red) in comparison to the binding observed with nonactivatable tris-NTA. Maximum binding of MBP-H6/10 after photoactivation for each interface is normalized to 100%.