Abstract
SUMMARY
Estrogen has an important role in the reconstruction of a new vascular network in the endometrium during each menstrual cycle; however, the underlying mechanisms are incompletely understood. Angiopoietin-1 (Ang-1) promotes vessel assembly, whereas Ang-2 and thrombospondin-1 (TSP-1) cause vessel breakdown. To determine the potential effect of estrogen on the expression of these angioregulatory factors in the endometrium, Ang-1, Ang-2, TSP-1, and Tie-2 receptor mRNA levels were assessed by real-time reverse transcriptase polymerase chain reaction in glandular epithelial and stromal cells isolated from the endometrium of ovariectomized baboons treated acutely with estradiol. Corresponding protein expression was assessed by immunocytochemistry and the proximity ligation assay (PLA) during advancing stages of the baboon menstrual cycle. Serum estradiol levels in ovariectomized baboons were 400 pg/ml within 4–6 hr of estradiol treatment. Ang-1 mRNA levels in glandular epithelial cells increased threefold (P < 0.01) within 4 hr of estradiol administration. In contrast, TSP-1 mRNA levels decreased four- to fivefold (P < 0.01) in endometrial glandular epithelial and stromal cells 4–6 hr after estradiol, whereas Ang-2 and Tie-2 expression was unaltered. Immunostaining for Ang-1 increased, TSP-1 decreased, and Ang-2 and Tie-2 were unaltered in the endometrium during the secretory compared with the proliferative phase of the cycle. Endometrial Ang-1 protein expression, quantified by PLA, increased 10-fold (P < 0.05) between the early proliferative and late proliferative/mid-secretory phases of the menstrual cycle in association with the rise in estrogen. In summary, estrogen induced a rapid, divergent, and cell-specific change in expression of angiostimulatory and angioinhibitory growth factors in the endometrium of the nonhuman primate.
INTRODUCTION
A new vascular system develops via angiogenesis and vascular remodeling in the endometrium during each menstrual cycle to support the cellular growth and differentiation required for blastocyst implantation (Brenner and Slayden, 1994; Albrecht and Pepe, 2003; Girling and Rogers, 2005; Jabbour et al., 2006, for reviews). Angiogenesis and vascular remodeling are orchestrated by coordinated interactions of key stimulatory and inhibitory factors. A widely recognized angiostimulatory factor, vascular endothelial growth factor (VEGF), stimulates endothelial cell proliferation, permeability, migration, and assembly into capillary tubes (Ferrara, 2004; Fan et al., 2008). Angiopoietin-1 (Ang-1), acting via the Tie-2 receptor, increases association of endothelial cells with pericytes/ vascular smooth muscle cells (VSMC) to remodel, stabilize, and mature newly formed blood vessels, whereas the angioinhibitory factors, Ang-2 and thrombospondin-1 (TSP-1), act to disassemble blood vessels (Hanahan, 1997; Maisonpierre et al., 1997; Yancopoulos et al., 2000; Visconti et al., 2002; Eklund and Olsen, 2006; Davis and Senger, 2008; Augustin et al., 2009). Thus, Ang-2, by inhibiting Tie-2 receptor signal transduction, elicits endothelial instability thereby causing vessel breakdown (Maisonpierre et al., 1997), whereas TSP-1 exerts antagonistic effects on vascular endothelial cell proliferation, migration, and assembly into microvessels (Iruela-Arispe et al., 1996; Iruela-Arispe and Dvorak, 1997; Nör et al., 2000).
VEGF, Ang-1, Ang-2, and TSP-1 mRNA and protein have been localized by in situ hybridization and immunocyto-chemistry in glandular epithelial, stromal and vascular cells of the human (Charnock-Jones et al., 1993; Iruela-Arispe et al., 1996; Shifren et al., 1996; Torry et al., 1996; Krikun et al., 2000; Hirchenhain et al., 2003; Saito et al., 2007) and rhesus monkey (Nayak and Brenner, 2002; Nayak et al., 2005) endometrium. Human endometrial microvascular endothelial cells (Möller et al., 2001) and pericytes/VSMC (Metheny-Barlow et al., 2004) express the Tie-2 receptor.
Despite the importance of VEGF, Ang-1/-2, and TSP-1 in promoting endometrial angiogenesis and vessel remodeling, relatively little is known about their coordinated regulation in the uterus. Estradiol upregulates endometrial VEGF expression in vivo in the rat (Cullinan-Bove and Koos, 1993; Hyder et al., 2000), sheep (Reynolds et al., 1998), and baboon (Niklaus et al., 2003; Albrecht et al., 2003; Aberdeen et al., 2008) and in vitro in the human (Charnock-Jones et al., 1993; Shifren et al., 1996; Huang et al., 1998). Little is known about the control of Ang-1 and the other angioregulatory factors in the endometrium, although glandular epithelial Ang-1 mRNA and protein levels in the rhesus monkey were increased by chronic administration of estradiol and progesterone in levels which mimicked the proliferative and secretory phases of the menstrual cycle (Nayak et al., 2005). However, chronic administration of estradiol results in cell differentiation, making it difficult to assess whether changes in expression of angioregulatory growth factors reflect direct or indirect effects of estrogen. Therefore, in this study we: (1) quantified the mRNA levels for Ang-1/-2, TSP-1, and Tie-2 in glandular epithelial and stromal cells isolated from the endometrium of ovariectomized baboons treated acutely with estradiol to assess the potential rapid direct impact of estrogen on their expression; (2) determined the immunocytochemical localization of Ang-1/-2, TSP-1, and Tie-2 in the endometrium of baboons during the proliferative and secretory phases of normal menstrual cycle, and (3) used the proximity ligation assay (PLA), a new immunocytochemistry-polymerase chain reaction (PCR)-based assay, to quantify Ang-1 protein expression in situ in the endometrium of baboons during the early and late proliferative and mid-secretory phases of the menstrual cycle.
RESULTS
Serum Estradiol Levels in Ovariectomized Baboons Treated Acutely with Estradiol
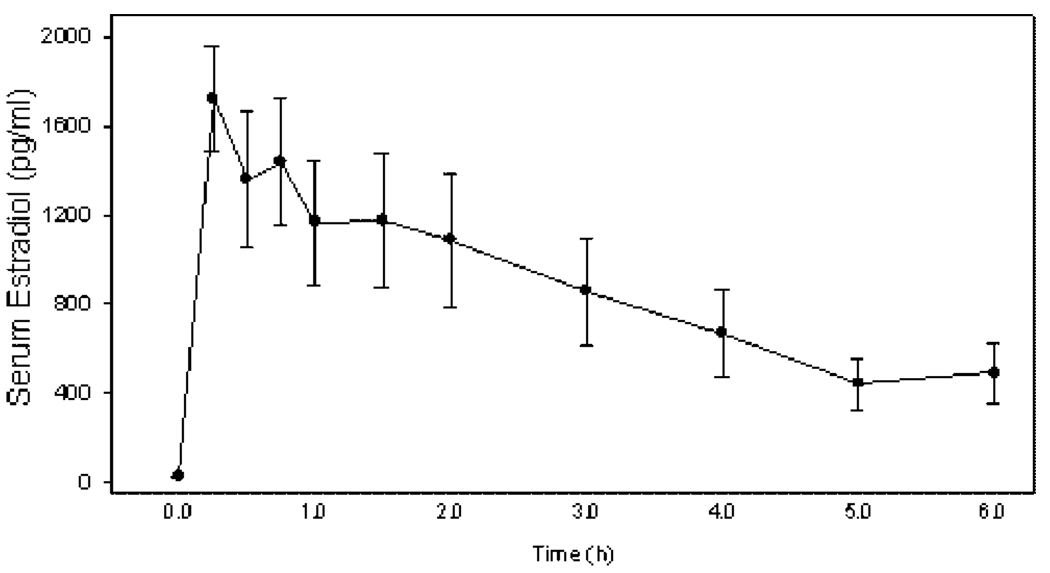
Peripheral serum estradiol levels in ovariectomized baboons were increased from less than 20 pg/ml prior to estrogen administration (i.e., 0 hr) to over 1,500 pg/ml within 15 min of estradiol treatment (Fig. 1). Serum estradiol concentrations then declined to approximately 400 pg/ml within 4–6 hr, a level approximating that of the normal mid-cycle estrogen peak (Albrecht et al., 1981).
Figure 1.
Serum estradiol levels (means ± SE) in ovariectomized baboons after administration of estradiol (bolus intravenous injection at 1 µg/kg body weight and subcutaneous insertion of three SILASTIC capsules containing estradiol) at time 0 hr (n = 11 baboons).
Endometrial Ang-1, Ang-2, Tie-2, and TSP-1 mRNA Levels in Ovariectomized Baboons Treated Acutely With Estradiol
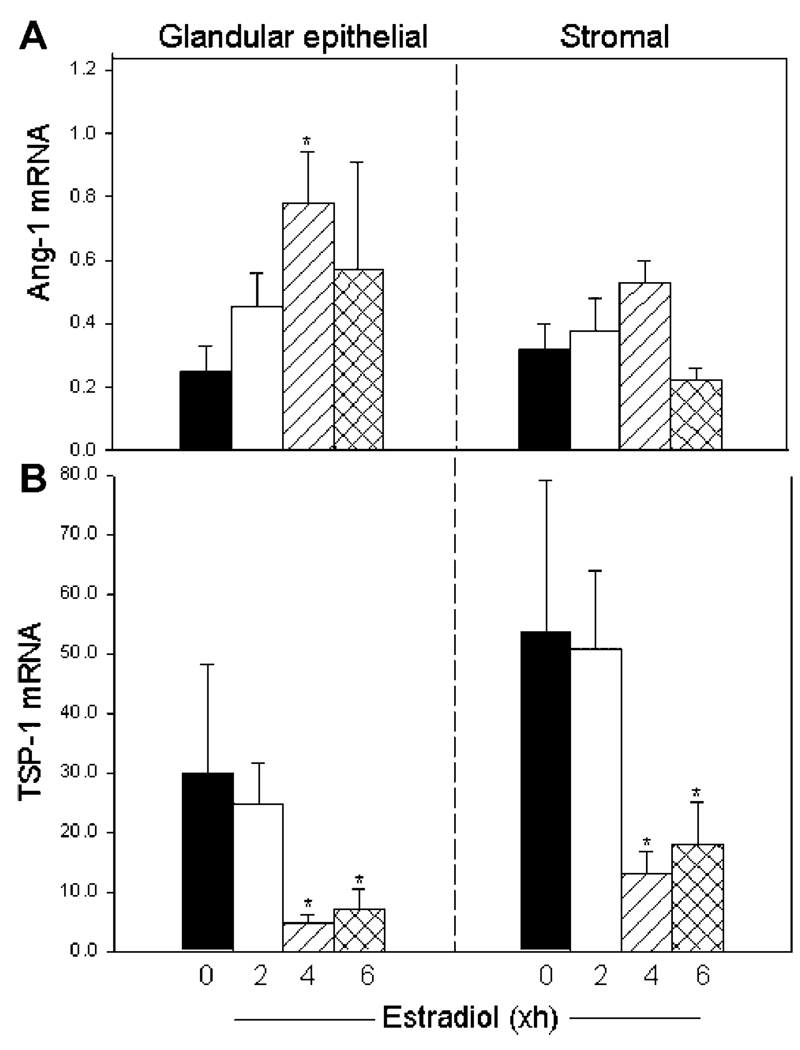
Ang-1 mRNA levels (expressed as a ratio of 18S rRNA)in glandular epithelial cells increased over threefold (P < 0.01) from 0.25 ± 0.08 at time 0 hr to 0.78 ± 0.16 within 4 hr of estrogen administration (Fig. 2A). Ang-1 mRNA levels in stromal tissue increased from 0.32 ± 0.11 at time 0 hr to 0.53 ± 0.07 at 4 hr after estrogen treatment (Fig. 2A), although these values were not significantly different.
Figure 2.
Endometrial angiopoietin-1 (Ang-1; A)andthrombospondin-1 (TSP-1; B) mRNA levels (means ± SE, corrected for 18S rRNA) determined by real-time reverse transcriptase-polymerase chain reaction in glandular epithelial and stromal cells isolated by laser capture microdissection from ovariectomized baboons (n = 11) treated at time 0 hr with estradiol as detailed in the legend of Figure 1. *Ang-1 mRNA levels different (P < 0.01) from values at time 0hr (ANOVA and Newman–Keul’s multiple comparison test). *TSP-1 mRNA levels collectively at 4 and 6hr different (P < 0.03, glands; P < 0.01, stroma) from values at 0 or 2 hr (Mann–Whitney test).
Prior to estradiol treatment, TSP-1 mRNA was expressed in levels over 100-fold greater than those of Ang-1 mRNA (Fig. 2). In contrast to the increase in Ang-1 mRNA expression after estradiol treatment, TSP-1 mRNA levels at 0 hr in glandular epithelial (29.84 ± 19.23) and stromal (53.58 ± 25.60) cells (Fig. 2B), although unaltered at 2 hr, declined to values at 4–6 hr after estradiol administration that were approximately five- (P < 0.03) and fourfold (P < 0.01) lower, respectively, than before treatment.
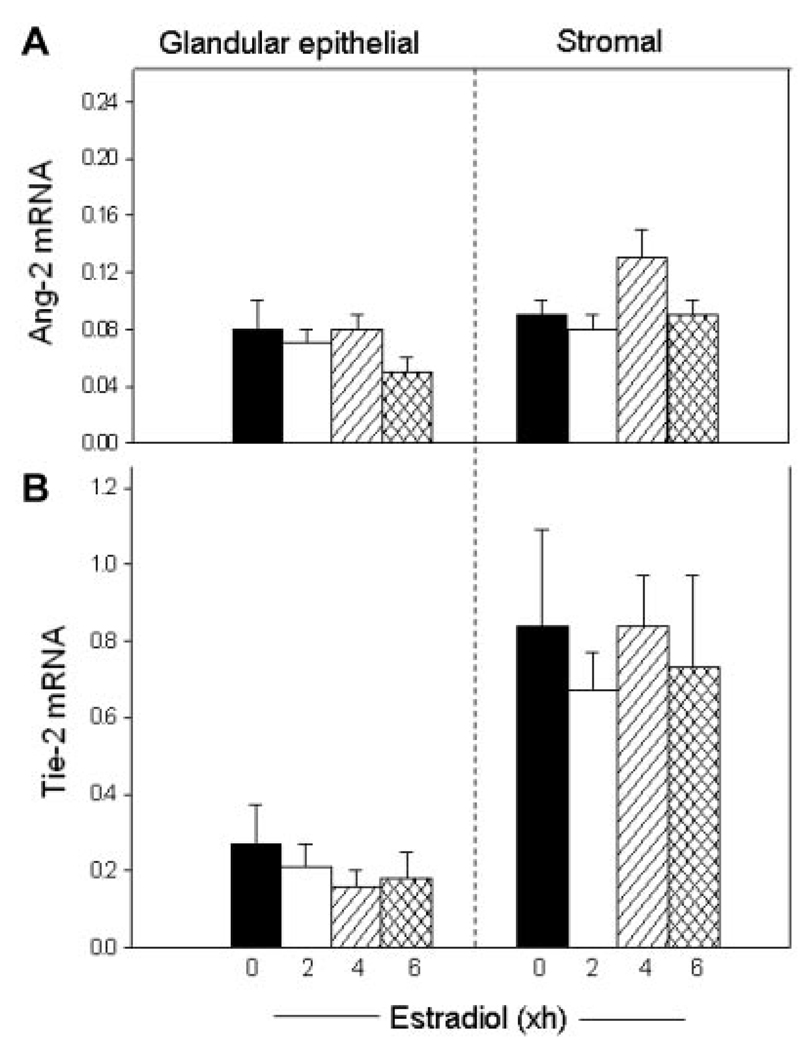
Endometrial Ang-2 mRNA levels were considerably lower than those of Ang-1 in both the glands and stroma, and were not changed by estrogen administration in either endometrial cell fraction (Fig. 3A). Tie-2 mRNA was expressed in high level in the endometrial stromal cell fraction, which is comprised of vascular and avascular tissues, and in lower level in the glands, but also was not altered in either location by acute estrogen treatment of baboons (Fig. 3B).
Figure 3.
Angiopoietin-2 (Ang-2; A) and Tie-2 (B) mRNA levels (ratio to 18S rRNA) in glandular epithelial and stromal cells of the estradiol-treated baboons in which Ang-1 and thrombospondin-1 mRNA levels are shown in Figure 2.
Immunocytochemistry of Endometrial Ang-1, Ang-2, Tie-2, and TSP-1 Protein During the Baboon Menstrual Cycle
Serum estradiol levels were at or below minimum level of RIA detection (i.e., 20 pg/ml) in the early proliferative phase, but increased to 102 pg/ml in the late proliferative phase and to 51 pg/ml in the mid-secretory phase of the baboon menstrual cycle.
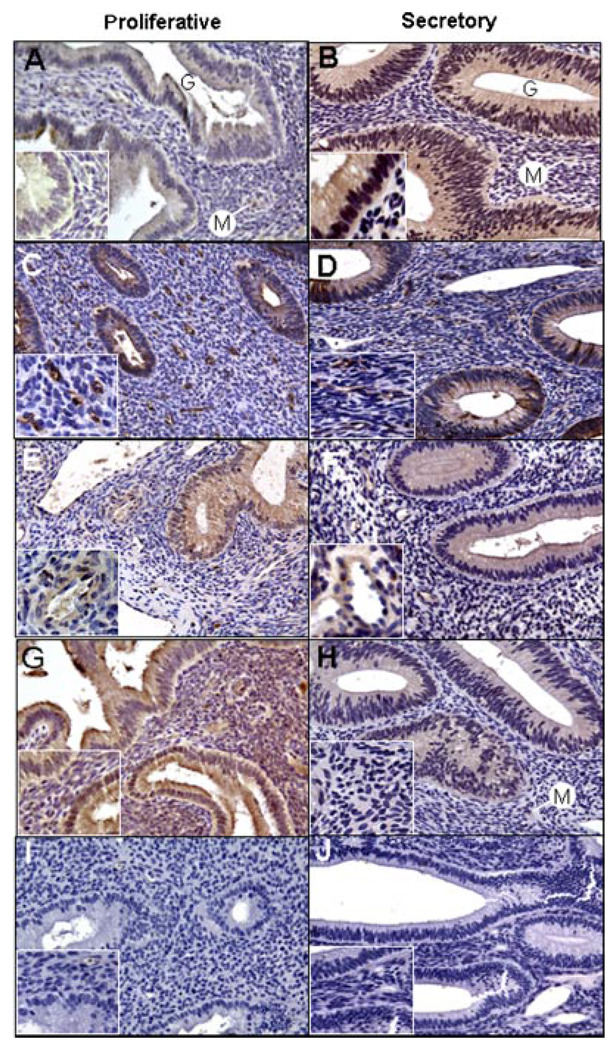
During the early proliferative phase (Fig. 4A), Ang-1 protein was localized by immunocytochemistry at very low levels in the glandular epithelium and stroma, and at moderate levels in the wall of microvessels (i.e., capillaries, arterioles, and venules). However, during the mid-secretory phase (Fig. 4B), the level of Ang-1 expression was abundant in the glandular epithelial and stromal cells, and remained moderate in microvascular cells. Ang-2 protein was expressed in relatively low abundance in the glands and stroma during both the proliferative (Fig. 4C) and secretory (Fig. 4D) phases of the baboon menstrual cycle. Ang-2 immunolabeling, however, was robust within microvessel cells in both the proliferative (Fig. 4C, insert) and secretory (Fig. 4D, insert) phases.
Figure 4.
Representative immunocytochemical localization (brown precipitate) of angiopoietin-1 (Ang-1; A, B), Ang-2 (C, D), Tie-2 receptor (E, F), and thrombospondin-1 (G, H) in the endometrium during the early (A, G) and late (C, E) proliferative and mid-late secretory (B,D,F,H) phases of the baboon menstrual cycle; (I) replacement of primary antibody with goat immunoglobulin G; (J) replacement of secondary anti-goat immunoglobulin in the Tie-2 reaction with secondary anti-mouse immunoglobulin. G, gland; M, microvessel. Final approximate magnification 200× in each panel, 640× in the inserts of panels A and B, and 400× in the remaining inserts.
The Tie-2 receptor was abundantly expressed in endothelial and/or VSMC of small blood vessels in the baboon endometrial stroma during both the proliferative (Fig. 4E, insert) and secretory (Fig. 4F, insert) phases. However, Tie-2 immunostaining was moderate in the glands and light in nonvascular cells of the stroma at these two stages of the menstrual cycle.
TSP-1 protein was abundantly expressed in stromal cells, and moderately expressed in glandular epithelial cells in the proliferative phase (Fig. 4G). The level of TSP-1 immunoreactivity in these two locations, particularly in the stromal cells, was decreased in the secretory (Fig. 4H, insert) when compared with the proliferative (Fig. 4G, insert) phases. TSP-1 was only lightly expressed in the walls of the endometrial microvessels during the proliferative and secretory phases of the baboon menstrual cycle.
Endometrial Ang-1 Protein Expression Assessed by PLA During the Menstrual Cycle
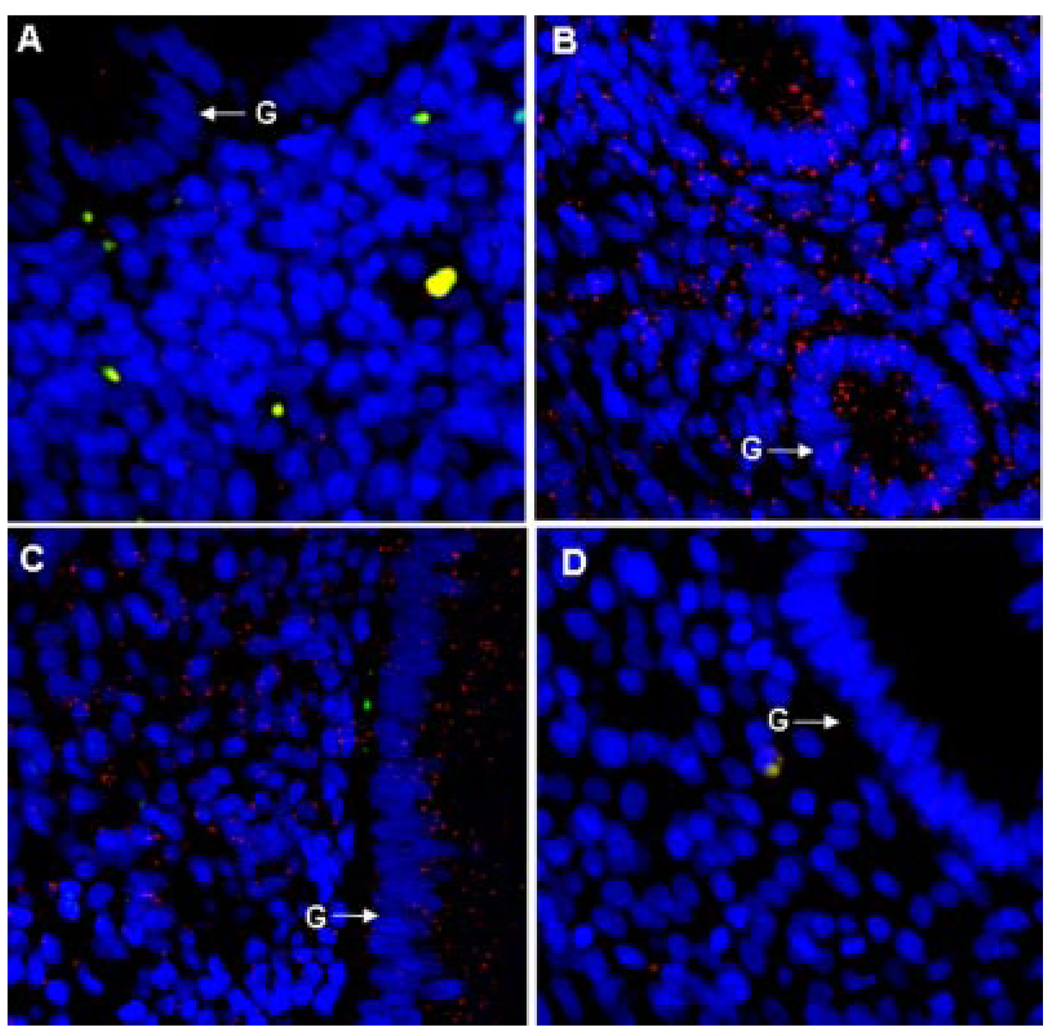
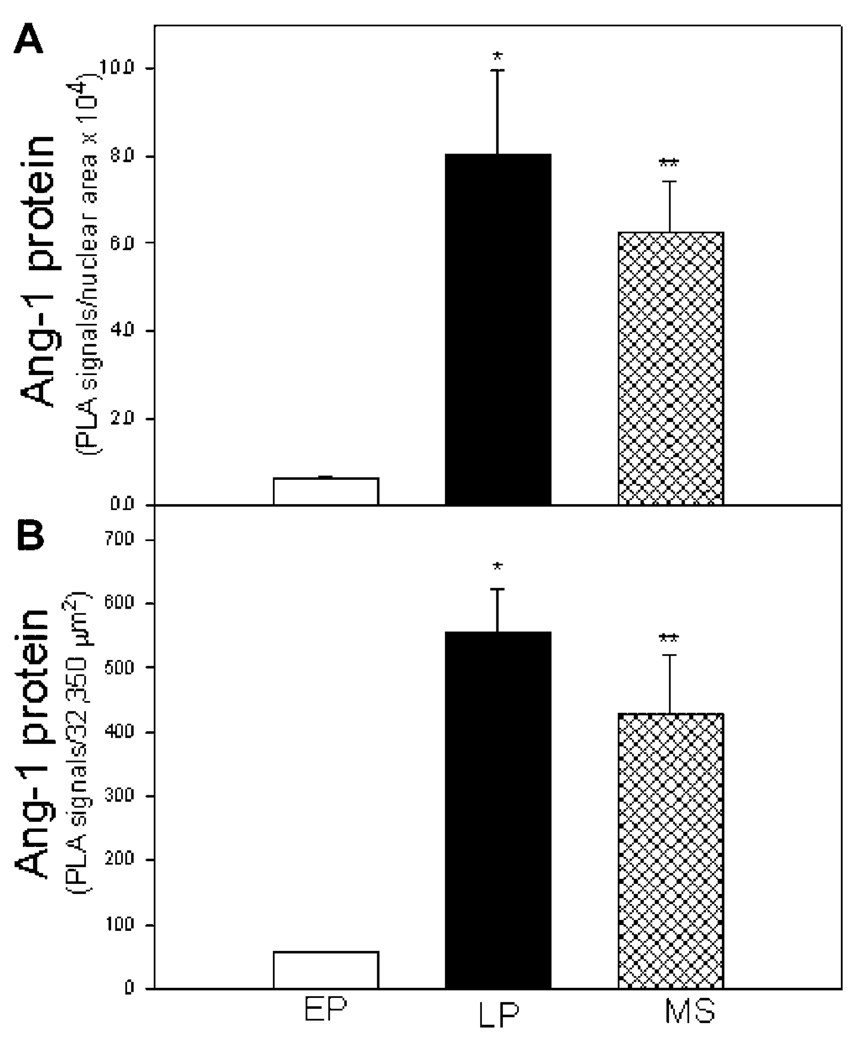
Ang-1 protein expression, detected by red immunofluorescent PLA signals/dots, appeared very low in the endometrium in the early proliferative phase (Fig. 5A) but was abundant in regions overlaying the glandular epithelial and stromal cells of the endometrium during the late proliferative (Fig. 5B) and mid-secretory (Fig. 5C) phases of the menstrual cycle. Thus, endometrial Ang-1 protein expression, quantified by PLA and Image J software analysis, increased approximately 10-fold from 0.65 ± 0.04 PLA signals per nuclear area × 104 (Fig. 6A) and 57 ± 10 PLA signals per 32,350 µm2 endometrial area (Fig. 6B) in the early proliferative phase to 8.04 ± 1.93 and 555 ± 68, respectively, in the late proliferative phase (P < 0.01) and to 6.27 ± 1.28 and 429 ± 92, respectively, in the mid-secretory phase (P < 0.05) of the menstrual cycle.
Figure 5.
Angiopoietin-1 protein expression assessed by the proximity ligation assay (PLA) in the endometrium during the early proliferative (A), late proliferative (B), and mid-secretory (C) phases of the baboon menstrual cycle. (D) Negative control with omission of primary Ang-1 antibody. Each red PLA signal/dot represents a single molecule of Ang-1 protein detected by primary Ang-1 antibody tagged with secondary antibody conjugated to fluorescently labeled oligonucleotide. Nuclei are labeled blue and red blood cells autofluoresce as yellow. G, glandular epithelium.
Figure 6.
Angiopoietin-1 protein expression quantified by the proximity ligation assay (PLA) and Image J software (mean ± SE; PLA signals/nuclear area × 104, panel A; or per 32,350 µm2 endometrial area, panel B) in the endometrium during the early proliferative (EP, n = 3 baboons), late proliferative (LP, n = 3), and mid-secretory (MS, n = 3) phases of the baboon menstrual cycle. Values are different (*P < 0.01 or **P< 0.05) from EP (ANOVA and Neuman–Keul’s multiple comparison test).
DISCUSSION
This study shows that the mRNA levels for Ang-1 were increased in endometrial glandular epithelial cells, for TSP-1 were decreased in glandular epithelial and stromal cells, and for Ang-2 and Tie-2 were unchanged in the endometrium of ovariectomized baboons treated acutely with estradiol. Because the changes in endometrial Ang-1 and TSP-1 mRNA expression occurred within 4 hr of estradiol administration, it is likely that estrogen elicited a direct cell-specific effect on transcription of these angioregulatory growth factors. The promoter region of Ang-1 contains putative estrogen-responsive elements (Lee et al., 2008). Although we have previously shown that acute estradiol administration rapidly increased endometrial VEGF expression in the baboon (Albrecht et al., 2003; Aberdeen et al., 2008), this study is the first to show that estrogen also elicits a rapid cell-specific increase in Ang-1 and decrease in TSP-1 expression in vivo in the endometrium of the nonhuman primate.
This study also showed that the immunolocalization of protein for Ang-1 appeared to increase, and for TSP-1 to decrease, in the glands and stroma during the estrogen- and progesterone-dominated secretory compared with the estrogen-deficient early proliferative phase of the baboon menstrual cycle, although assessment of protein expression by immunostaining is not quantitative. However, using PLA to quantify protein in situ, this study further shows that endometrial Ang-1 protein expression was approximately 10-fold greater in the late proliferative and mid-secretory phases, when estradiol and estradiol plus progesterone levels become elevated, than in the very early proliferative phase of the menstrual cycle when estradiol levels are low. The increase in endometrial Ang-1 protein expression, in association with the menstrual cycle-dependent rise in estradiol levels, is consistent with the increase in Ang-1 mRNA levels induced by acute estrogen administration in ovariectomized baboons. The changes in endometrial Ang-1 and TSP-1, and absence of changes in Ang-2 and Tie-2 expression in baboons are also consistent with other recently published studies that used very different experimental paradigms. Thus, Nayak et al. (2005) reported that mRNA and protein levels for Ang-1 were increased in the glandular epithelium, but Tie-2 levels remained unchanged in the VSMC of the endometrium of ovariectomized rhesus monkeys after chronic administration of estradiol was used to mimic the late proliferative phase, and estradiol plus progesterone was used to mimic the secretory phase of the menstrual cycle compared with hormone deprivation. Estradiol also increased Ang-1, but not Tie-2, mRNA levels in vitro in human (Mirkin and Archer, 2007) and in vivo in sheep (Johnson et al., 2006) endometrial cells. Moreover, Ang-2 mRNA levels were not altered by the addition of estradiol to cultures of human endometrial stromal or endothelial cells (Krikun et al., 2004) or in the endometrium of women treated with estradiol during in vitro fertilization embryo transfer (Lédée et al., 2006). Endometrial glandular epithelial Ang-1 protein and/or mRNA expression also appeared greater, whereas Ang-2 and Tie-2 expression appeared similar, in the secretory versus the proliferative phases of the human menstrual cycle (Hirchenhain et al., 2003; Saito et al., 2007). Consistent with the results for TSP-1 in baboons of this study, estrogen receptor antagonist increased TSP-1 mRNA levels in endometrial cells (Navarro et al., 2003) and estradiol decreased TSP-1 expression in human umbilical vein endothelial cells (Sengupta et al., 2004). In apparent contrast to the results for TSP-1 expression in baboons, endometrial TSP-1 mRNA and protein expression was elevated during the secretory compared with the proliferative phases of the human menstrual cycle and increased by progesterone but not estradiol in human endometrial cell cultures (Iruela-Arispe et al., 1996; Seki et al., 2001). Although the underlying reason(s) for the apparent difference in endometrial TSP-1 expression in the latter human study and this baboon study is unknown, additional study of baboons treated with estradiol and/or progesterone is needed to clarify the respective roles of these two steroid hormones in regulating endometrial expression of TSP-1, and the other angioregulatory factors.
The physiological relevance of the estrogen- and menstrual cycle-dependent differential change in expression of angiostimulatory and angioinhibitory growth factors within the nonhuman primate endometrium shown in our recent and present studies requires further investigation. However, estrogen promoted vascular endothelial cell proliferation in the rodent and rhesus monkey uterus via VEGF-mediated effects (Nayak and Brenner, 2002; Hastings et al., 2003; Heryanto et al., 2003). We have shown that VEGF also mediated the estrogen-induced increase in baboon endometrial microvessel interendothelial cell permeability (Aberdeen et al., 2008), an early step in angiogenesis (Nagy et al., 2008). Ang-1 stimulates coupling of periendothelial cells with endothelial cells to remodel, mature, and stabilize newly formed blood vessels (Maisonpierre et al., 1997; Thurston et al., 1999; Yancopoulos et al., 2000; Augustin et al., 2009). Therefore, we propose that the increase in endometrial VEGF and Ang-1, and the decline in TSP-1 expression elicited after estradiol treatment and with advancing stages of the menstrual cycle in primates underlie the fundamentally important well-established role for ovarian estrogen in regulating growth, reconstruction, and remodeling of the endometrial blood vessel network.
In summary, the transition from early proliferative to late proliferative and secretory phases of the baboon menstrual cycle are coordinated with an increased expression of Ang-1, TSP-1 decreased, and no change in Ang-2 and Tie-2 levels in glandular epithelial, stromal, and/or microvessel cells of the endometrium after acute administration of estradiol to ovariectomized baboons in the late proliferative and secretory phases, compared with the early proliferative phase of the baboon menstrual cycle. Consequently, estrogen induced a rapid, divergent, and cell-specific change in expression of angiostimulatory and angioinhibitory growth factors in the endometrium of the nonhuman primate.
MATERIALS AND METHODS
Animals
Adult female baboons (Papio anubis), originally obtained from the Southwest Foundation for Biomedical Research (San Antonio, TX), exhibiting regular menstrual cycles and weighing 12–15 kg were used in this study. Baboons were housed individually in large primate cages in air-conditioned rooms, 12-hr light:12-hr dark cycle, and received primate chow (Teklad-Harlan, St. Louis, MO) and fresh fruit twice daily and water ad libitum. Animals were cared for and used strictly in accordance with US Department of Agriculture regulations and the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (National Academy Press, 1996). The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
In the first experiment, we determined the effect of acute estrogen administration on endometrial mRNA levels of the angioregulatory growth factors in 11 baboons that had been bilaterally ovariectomized 60 days earlier to remove the principal source of estrogen and progesterone, as described previously (Albrecht et al., 2003). During the 5 days immediately preceding the acute study, ovariectomized baboons were injected subcutaneously (s.c.) daily with the highly specific aromatase inhibitor letrozole (4,4′ -[1,2,3-triazyol-1-yl-methylene]-bis-benzonitrite; Novartis Pharma AG, Basel, Switzerland) at a dosage of 0.5 mg/0.25 cc sesame oil to suppress potential aromatization in nonovarian sites. At 08:00 hr on the day of acute estrogen treatment, baboons were anesthetized with isoflurane, placed in a supine position on a 37°C warming pad on a surgical table, and administered 5% dextrose (25 ml/hr) via a peripheral saphenous vein. A midline 6-cm abdominal incision was then made for subsequent endometrial biopsy, and at time 0 hr the baboons were administered a bolus of 17β-estradiol (1.0 µg/kg body weight; Sigma, St. Louis, MO; in 0.5 ml ethanol:normal saline injected into an antecubital vein) plus three SILASTIC capsules of estradiol (Dow Corning, Midland, MI; s.c., 5mm diameter, 6 cm length) to elicit a rapid surge and sustained release of hormone. Blood samples (2 ml) were obtained via the peripheral saphenous vein catheter periodically during the study period for determination of serum estradiol concentrations by RIA (Albrecht et al., 2000).
Single 5-mm diameter core tissue biopsies (Acu-Punch, Acuderm, Inc., Ft. Lauderdale, FL) were obtained 0, 2, 4, and 6 hr after estrogen administration from the uterine fundus, alternating from anterior and posterior surfaces, extending transmurally from the outer surface to lumen. The tissue samples were sectioned longitudinally, embedded in cryomolds filled with OCT medium (Sakura Finetek USA, Inc., Torrance, CA), frozen on dry ice, and stored at −80°C for subsequent mRNA analysis in glandular epithelial and stromal cells isolated by laser capture microdissection (LCM).
In the second experiment, Ang-1/-2, Tie-2, and TSP-1 were localized by immunocytochemistry in endometrial tissue samples obtained after laporatomy from isoflurane-anesthetized baboons during the early or late proliferative (n = 3 baboons) and mid-late secretory (n = 3) phases of the cycle. In the last experiment, Ang-1 protein expression was quantified by PLA in endometrial tissue obtained after laparotomy from baboons in the very early proliferative (n = 3), late proliferative (n = 3), and mid-secretory (n = 3) phases of the normal menstrual cycle. The stages of the menstrual cycle were determined by daily recording of the pattern of tumescence and detumescence of the external sex skin (Albrecht et al., 1981). Endometrial tissue was fixed in 10% phosphate-buffered saline (PBS) with formalin for 24 hr and embedded in paraffin for both immunocytochemistry and PLA.
LCM of Endometrial Cells
Glandular epithelial and stromal cells were isolated from the endometrium by LCM as described previously (Niklaus et al., 2003). Briefly, serial sections (8 µm) of each uterine biopsy were cut longitudinally (to include endometrium and myometrium) via a Jung Frigocut 2800E cryostat at −20°C (Leica Corp., Deerfield, IL) and mounted onto glass slides. The sections were immediately fixed in 70% ethanol, lightly stained with hematoxylin, and dehydrated in 100% ethanol then xylene. Slides were air-dried and transferred to a desiccator at room temperature, and an Arcturus PixCell II LCM system equipped with an Olympus Corp. microscope (Arcturus Engineering, Inc., Mountain View, CA) was then used to capture glandular (but not luminal) epithelial and stromal cells randomly from both the basalis and functionalis zones of the endometrium. A laser power of 40 mW, duration of 1.5–2.5 msec, and spot size of 7.5–15 µm for glandular epithelium and 15–30 µm for stroma were used for cell isolation. Captured cells were mixed with lysis buffer (RNeasy, QIAGEN, Valencia, CA), microcentrifuged, stored in lysate buffer overnight at −80°C, and RNA was extracted within 72 hr.
RT-PCR of Ang-1, Ang-2, Tie-2, and TSP-1 mRNA
Oligonucleotide primers were designed using LightCycler probe design software (Roche Diagnostics Corp., Penzberg, Germany).
Ang-1 primers
Oligonucleotide primers were based on the Ang-1 human gene sequence (NCBI database accession #U83508) and supplied by TIB MOLBIOL (Adelphia, NJ): 5′-GGGGGAGGTTGG-ACTGTAAT-3′ (positions 1270–1289) and 5′-AGGGCACATTTG-CACATACA-3′ (positions 1631–1612).
TSP-1 primers
Oligonucleotide primers were based on the TSP-1 human gene sequence (NCBI database accession #M25631) and supplied by Invitrogen (Carlsbad, CA): 5′-GCCTGATGACAAG-TTCCAAGA-3′ (positions 309–329) and 5′-GTCTCTGGTGAAGA-CGCTTTG-3’ (positions 669–649).
Ang-2 primers
Oligonucleotide primers were based on the Ang-2 human gene sequence (NCBI database accession #AF004327) and supplied by Invitrogen (Carlsbad, CA): 5′-GGATCTGGG-GAGAGAGGAAC-3′ (positions 51–70) and 5′-CTCTGCACCGA-GTCATCGTA-3′ (positions 585–566).
Tie-2 primers
Oligonucleotide primers were based on the Tie-2 human gene sequence (NCBI database accession #L06139 and supplied by TIB MOLBIOL (Adelphia, NJ): 5′-GGCTGGCCGC-TACCTACTAA-3′ (positions 1268–1287) and 5′-GCTACTGAGA-AATGATCCGTATGGTT-3′ (positions 1368–1343).
18S rRNA primers
Oligonucleotide 18S rRNA primers were based on the human gene sequence (NCBI database, accession #M10098): 5′-TCAAGAACGAAAGTCGGAGG-3′ (positions 1126–1145) and 5′-GGACATCTAAGGGCATCACA-3′ (positions 1614–1595).
RT and real-time PCR
Reverse transcription (RT) of total RNA from LCM isolates was performed according to manufacturer’s directions (Invitrogen). A 13-µl reaction volume containing 1 mM each of deoxy (d)-ATP, dCTP, dGTP, and dTTP, 1X RT buffer, 250 ng of random primers, and total RNA was incubated at 65°C for 5min and on ice for 1 min. Reaction buffer, 200 U Superscript III RT (Invitrogen) and 40 U RNAguard (Amersham Pharmacia Biotech, Piscataway, NJ) were incubated at 25°C for 5 min and 50°C for 60 min. The RT reaction was terminated by heat inactivation at 70°C for 15 min and cooled to 4°C.
mRNA levels were quantified via a LightCycler real-time PCR unit (Roche Diagnostics Corp.) using Fast Start DNA Master SYBR Green I Kit for PCR. A 19-µl reaction mix containing either target mRNA- or 18S rRNA-specific primers was combined with 1 µl aliquot of RT reaction for a final volume of 20 µl. The reaction profile consisted of denaturation at 95°C for 8 min, 40 cycles of amplification (95°C for 5 sec, 52°C for 5 sec, and 72°C for 16 sec) and product formation measured and displayed in real time. Efficiency-corrected calibrator-normalized relative quantification was performed with LightCycler version 4 software using premade standard curves specific for each product to correct for differences in the efficiencies of target and reference genes, and an in-run calibrator to normalize quantification. Specificity of the products was confirmed by melting curve analysis, agarose gel electrophoresis, and inclusion of negative controls with no template or no RT in the reaction.
Immunocytochemistry
For antigen retrieval and optimization of specificity, endometrial tissue sections were boiled in 0.01 M Na citrate, pretreated with protease (Biomeda, Foster City, CA) for 5 min at room temperature, incubated in H2O2 to inhibit endogenous peroxidase and blocked with serum-free protein block (Dako Corp., Carpenteria, CA). Tissues were incubated overnight at 4°C with goat polyclonal antibodies to human Ang-1, Ang-2, and Tie-2 (1:80, 1:160, 1:50 dilution, respectively, all from R&D Systems, Inc., Minneapolis, MN) and mouse monoclonal antibody to TSP-1 (1:75 dilution, gift from Dr. J. Murphy-Ullrich, University of Alabama, Birmingham, AL).
Tissues were incubated for 1 hr at room temperature with biotinylated anti-goat or anti-mouse immunoglobulins (Vector Laboratories, Inc., Burlingame, CA) and for 1 hr with an avidin–biotin–peroxidase complex (ABC Elite; Vector Laboratories). Tissue sections were developed using diaminobenzidine (Sigma) and lightly counterstained with hematoxylin.
Negative controls included replacement of the primary antibody with nonimmune immunoglobulin G or substitution of species-inappropriate secondary immunoglobulin (Dako).
Proximity Ligation Assay (PLA)
PLA was performed using reagents and directions supplied by Olink Bioscience (Uppsala, Sweden). Paraffin-embedded endometrial tissue sections (5-µm thick) were subjected to antigen retrival and protein block, and incubated overnight at 4°C with goat polyclonal primary Ang-1 antibody (R&D Systems, Inc.) as described before. Tissue was then incubated for 1 hr at room temperature with rabbit anti-goat immunoglobulins (Vector Laboratories) and then for 2hr at 37°C in a preheated humidity chamber with PLA probes consisting of two secondary anti-rabbit antibodies each tagged with an oligonucleotide (diluted 1:5 in PBS/5% NHS). A hybridization solution consisting of two oligonucleotide linkers, complementary to each PLA probe, was added to the tissue for 15 min at 37°C in a humidity chamber. Tissue sections were washed and incubated for 15 min at 37°C with a Duolink Ligation stock (supplied in the PLA kit and diluted 1:5 in water) containing ligase (diluted 1:40 in buffer) and for 90 min with Duolink polymerase (diluted 1:80 in amplification buffer). The ligation step connects the hybridization linkers to the PLA probe oligonucleotides, forming a complete circle connecting both antibodies, and the polymerization step uses rolling circle DNA amplification to generate a concatameric oligonucleotide product linked to the antibody complex. Following washing, the tissue was incubated for 60 min with a detection solution (diluted 1:5 in water), consisting of fluorescently labeled oligonucleotides which hybridize to the rolling circle amplification product, and Hoescht 33342 stain for detection of the nuclei. The tissue sections were then washed in decreasing concentrations of sodium citrate buffer containing 0.05% Tween 20, washed in 70% ethanol, briefly dried, and cover-slipped with the mounting media. Ang-1 protein red PLA signals were visualized by fluorescence microscopy (Nikon Eclipse E 1000, Tokyo, Japan) and image analysis software (IP Lab Scientific Imaging Processing, Scanalytics, Fairfax, VA). PLA signals were quantified using Image J software (v1.41o, N.I.H. open software) in a minimum of five randomly selected areas (32,350 µm2) of each endometrial sample. Negative controls for PLA included omission of the primary antibody, omission of PLA probe, and omission of goat immunoglobulin to create goat–rabbit species mismatch between primary antibody and PLA probes.
Statistical Analysis
Data were expressed as the means ± SE. Endometrial mRNA and protein levels were analyzed by ANOVA with post hoc comparisons of the means by Newman–Keul’s multiple comparison test or by the Mann–Whitney test.
ACKNOWLEDGMENTS
The authors appreciated the secretarial assistance of Mrs Wanda James with preparation of the figures and manuscript. This work was supported by NICHD/NIH through cooperative agreement U54 HD36207 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and by NIH Research Grant RO1 HD13294.
REFERENCES
- Aberdeen GW, Wiegand SJ, Bonagura TW, Jr, Pepe GW, Albrecht ED. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology. 2008;149:6076–6083. doi: 10.1210/en.2008-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Niklaus AL, Babischkin JS, Suresch DL, Pepe GJ. Acute temporal regulation of vascular endothelial growth/permeability factor expression and endothelial morphology in the baboon endometrium by ovarian steroids. J Clin Endocrinol Metab. 2003;88:2844–2852. doi: 10.1210/jc.2002-021546. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol. 2000;182:432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Haskins AL, Hodgen GD, Pepe GJ. Luteal function in baboons with administration of the antiestrogen ethamoxytriphetol (MER-25) throughout the luteal phase of the menstrual cycle. Biol Reprod. 1981;25:451–457. doi: 10.1095/biolreprod25.3.451. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. Steroid hormone regulation of angiogenesis in the primate endometrium. Front Biosci. 2003;8:d416–d429. doi: 10.2741/1001. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD. Cyclic changes in the primate oviduct and endometrium. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press, Ltd; 1994. pp. 541–569. [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. Identification and localization of alternatively spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: Rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology. 1993;133:829–837. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Girling JE, Rogers PA. Recent advances in endometrial angiogenesis research. Angiogenesis. 2005;8:89–99. doi: 10.1007/s10456-005-9006-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Licence DR, Burton GJ, Charnock-Jones DS, Smith SK. Soluble vascular endothelial growth factor receptor 1 inhibits edema and epithelial proliferation induced by 17betaestradiol in the mouse uterus. Endocrinology. 2003;144:326–334. doi: 10.1210/en.2002-220641. [DOI] [PubMed] [Google Scholar]
- Heryanto B, Lipson KE, Rogers PA. Effect of angiogenesis inhibitors on oestrogen-mediated endometrial endothelial cell proliferation in the ovariectomized mouse. Reproduction. 2003;125:337–346. doi: 10.1530/rep.0.1250337. [DOI] [PubMed] [Google Scholar]
- Hirchenhain J, Huse I, Hess A, Bielfeld P, DeBruyne F, Krüssel JS. Differential expression of angiopoietins 1 and 2 and their receptor Tie-2 in human endometrium. Mol Hum Reprod. 2003;9:663–669. doi: 10.1093/molehr/gag083. [DOI] [PubMed] [Google Scholar]
- Huang JC, Liu DY, Dawood MY. The expression of vascular endothelial growth factor isoforms in cultured human endometrial stromal cells and its regulation by 17beta-oestradiol. Mol Hum Reprod. 1998;4:603–607. doi: 10.1093/molehr/4.6.603. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Mäkelä S, Chiappetta C, Stancel GM. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108:785–790. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Dvorak HF. Angiogenesis: A dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997;78:672–677. [PubMed] [Google Scholar]
- Iruela-Arispe ML, Porter P, Bornstein P, Sage EH. Thrombospondin-1, an inhibitor of angiogenesis, is regulated by progesterone in the human endometrium. J Clin Invest. 1996;97:403–412. doi: 10.1172/JCI118429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HOD. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine. 2006;30:333–342. doi: 10.1007/s12020-006-0012-5. [DOI] [PubMed] [Google Scholar]
- Krikun G, Sakkas D, Schatz F, Buchwalder L, Hylton D, Tang C, Lockwood CJ. Endometrial angiopoietin expression and modulation by thrombin and steroid hormones: A mechanism for abnormal angiogenesis following long-term progestin-only contraception. Am J Pathol. 2004;164:2101–2107. doi: 10.1016/S0002-9440(10)63768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Schatz F, Finlay T, Kadner S, Mesia A, Gerrets R, Lockwood CJ. Expression of angiopoietin-2 by human endometrial endothelial cells: Regulation by hypoxia and inflammation. Biochem Biophys Res Commun. 2000;275:159–163. doi: 10.1006/bbrc.2000.3277. [DOI] [PubMed] [Google Scholar]
- Lédée N, Dubanchet S, Lombroso R, Ville Y, Chaouat G. Downregulation of human endometrial IL-18 by exogenous ovarian steroids. Am J Reprod Immunol. 2006;56:119–123. doi: 10.1111/j.1600-0897.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Lee YL, Liu Y, Ng PY, Lee KF, Au CL, Ng EH, Ho PC, Yeung WS. Aberrant expression of angiopoietins-1 and -2 and vascular endothelial growth factor-A in peri-implantation endometrium after gonadotrophin stimulation. Hum Reprod. 2008;23:894–903. doi: 10.1093/humrep/den004. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie 2 disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–230. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Archer DF. Effects of tibolone and its metabolites on angiopoietin-1, Tie-2 and tumor necrosis factor-alpha mRNA in Ishikawa cells. Implication for tibonone’s effects on the endometrium. Maturitas. 2007;57:338–346. doi: 10.1016/j.maturitas.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Möller B, Rasmussen C, Lindblom B, Olovsson M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod. 2001;7:65–72. doi: 10.1093/molehr/7.1.65. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Benjamin L, Zenh H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro FJ, Mirkin S, Archer DF. Effect of raloxifene, 17betaestradiol, and progesterone on mRNA for vascular endothelial growth factor isoforms 121 and 165 and thrombospondin-1 in Ishikawa cells. Fertil Steril. 2003;79:1409–1415. doi: 10.1016/s0015-0282(03)00350-9. [DOI] [PubMed] [Google Scholar]
- Nayak NR, Brenner RM. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab. 2002;87:1845–1855. doi: 10.1210/jcem.87.4.8413. [DOI] [PubMed] [Google Scholar]
- Nayak NR, Kuo CJ, Desai TA, Wiegand SJ, Lasley BL, Giudice LC, Brenner RM. Expression, localization and hormonal control of angiopoietin-1 in the rhesus macaque endometrium: Potential role in spiral artery growth. Mol Hum Reprod. 2005;11:791–799. doi: 10.1093/molehr/gah237. [DOI] [PubMed] [Google Scholar]
- Niklaus AL, Aberdeen GW, Babischkin JS, Pepe GJ, Albrecht ED. Effect of estrogen on vascular endothelial growth/permeability factor expression by glandular epithelial and stromal cells in the baboon endometrium. Biol Reprod. 2003;68:1997–2004. doi: 10.1095/biolreprod.102.011288. [DOI] [PubMed] [Google Scholar]
- Nör JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000;37:209–218. doi: 10.1159/000025733. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Kirsch JD, Kraft KC, Redmer DA. Time-course of the uterine response to estradiol-17beta in ovariectomized ewes: Expression of angiogenic factors. Biol Reprod. 1998;59:613–620. doi: 10.1095/biolreprod59.3.613. [DOI] [PubMed] [Google Scholar]
- Saito M, Sato Y, Watanabe J, Kuramoto H, Kaba S, Fukuda T. Angiogenic factors in normal endometrium and endometrial adenocarcinoma. Pathol Int. 2007;57:140–147. doi: 10.1111/j.1440-1827.2006.02071.x. [DOI] [PubMed] [Google Scholar]
- Seki N, Kodama J, Hashimoto I, Hongo A, Yoshinouchi M, Kudo T. Thrombospondin-1 and -2 messenger RNA expression in normal and neoplastic endometrial tissues: Correlation with angiogenesis and prognosis. Int J Oncol. 2001;19:305–310. doi: 10.3892/ijo.19.2.305. [DOI] [PubMed] [Google Scholar]
- Sengupta K, Banerjee S, Saxena NK, Banerjee SK. Thombospondin-1 disrupts estrogen-induced endothelial cell proliferation and migration and its expression is suppressed by estradiol. Mol Cancer Res. 2004;2:150–158. [PubMed] [Google Scholar]
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: Implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Torry DS, Holt VJ, Keenan JA, Harris G, Caudle MR, Torry RJ. Vascular endothelial growth factor expression in cycling human endometrium. Fertil Steril. 1996;66:72–80. [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Weigand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]