Figure 4.
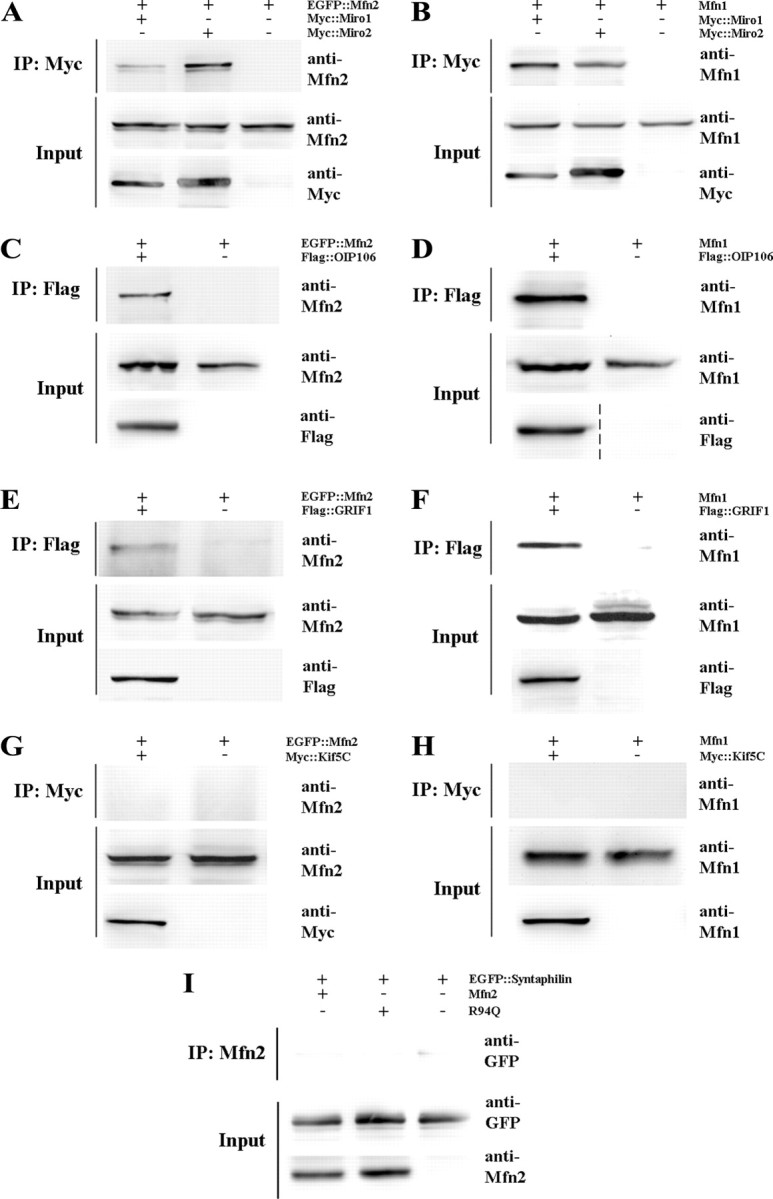
Mfn2 and Mfn1 interact with Miro and Milton proteins, key components of microtubule-based mitochondrial transport. HEK 293T cells were transiently transfected with the indicated epitope-tagged constructs, followed by immunoprecipitation (IP) and immunoblotting. A, B, Both Mfn2 and Mfn1 were able to interact with Miro1 and Miro2 by coimmunoprecipitation. The Mfn2:Miro2 interaction was consistently more robust than the Mfn2:Miro1 interaction, suggesting there may be selectivity for the formation of this complex. C–F, Mfn2 and Mfn1 interact with the Milton homologues OIP106 and GRIF1, proteins known to function as linkers between mitochondria and kinesins motors. G, H, Neither Mfn2 nor Mfn1 coimmunoprecipitated with Kif5C, indicating that they do not directly link mitochondria to kinesin. I, Additionally, Mfn2 was unable to interact with syntaphilin, an outer mitochondrial membrane protein that anchors mitochondria to microtubules.
