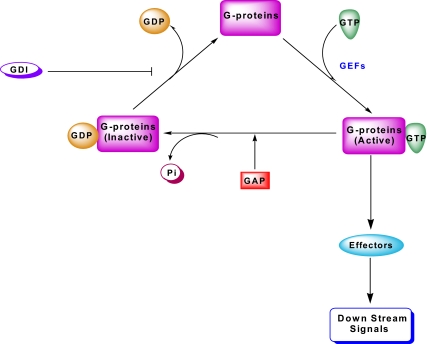
Figure 3.
Guanine nucleotide regulatory proteins/factors involved in the activation-deactivation cycle of G proteins. The small G proteins (e.g., Cdc42 and Rac1) in their GDP-bound (inactive) conformation remain associated with their respective GDIs. The principal role of GDIs is to prevent dissociation of GDP from the corresponding G protein. After the receipt of the appropriate signal, the G protein/GDI complex dissociates, thereby facilitating GTP/GDP exchange mediated by various guanine nucleotide exchange factors (GEFs). The GTP-bound, functionally active G protein, in turn, regulates its effector proteins and downstream signaling steps leading to cellular (de)activation. GTP bound to these G proteins is hydrolyzed by the GTPase activity intrinsic to the candidate G protein, to GDP yielding the inactive conformation of the G protein. Under specific conditions, the GTPase activity can be stimulated by additional regulatory factors, such as the GAP.