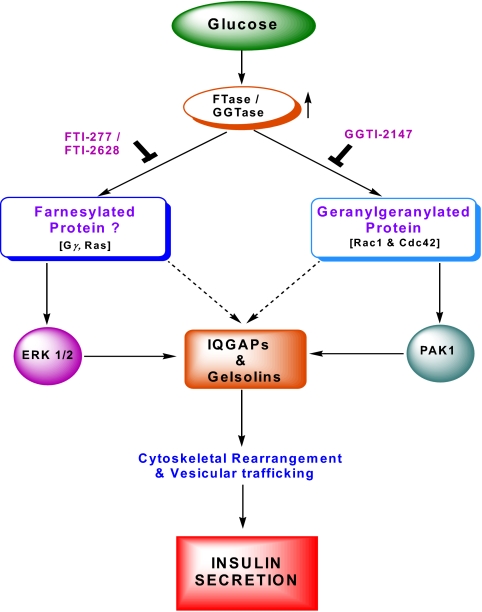
Figure 5.
A model for glucose-mediated activation of farnesylated and geranylgeranylated proteins leading to GSIS. Identification of ERK and PAK1 as target proteins. Glucose metabolism leads to the activation of islet endogenous FTases and GGTases culminating in the activation of farnesylated and geranylated proteins, respectively. Such conclusions were reached by pharmacological and molecular biological approaches. It appears that activation of a yet to be identified farnesylated protein(s) is necessary for glucose-mediated activation of ERK and subsequent effects on insulin gene transcription and insulin secretion. On the other hand, glucose-mediated activation of Cdc42 and Rac1 leads to regulation of PAK1 activity. Potential phosphoprotein substrates for PAK1 have not been identified in the β-cell up until now, but could include Rho-GDI. The activation of ERK and PAK (and other effector proteins) facilitate reorganization of the actin cytoskeletal architecture leading to translocation and fusion of insulin granules with the plasma membrane and release of insulin. An active involvement of IQGAPs and gelsolins in these signaling pathways in the organization of the cytoskeletal architecture is also proposed.