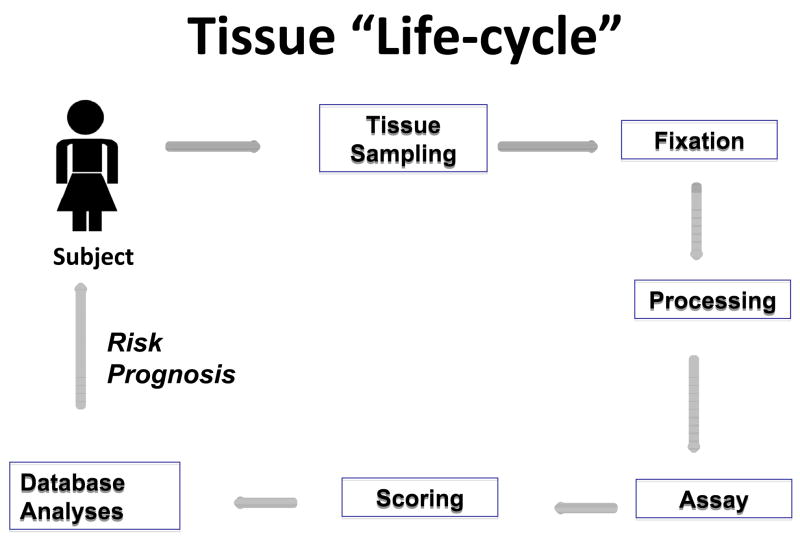
Figure 1.
The patients for whom research tissues are available in epidemiologic studies are a function of multiple factors related to diagnosis and treatment. These practices may vary between populations, geographic regions and calendar period and by patient and tumor features. Practices of tissue sampling and preparation for diagnosis may vary with the type of procedure used to collect the sample, the clinical context and institutional protocols. All of the above may affect assay performance. In large studies, efforts to standardize, optimize and automate performance and scoring of assays are important to control costs, speed completion of studies and minimize variability. Computer infrastructure is important throughout the “cycle”, to provide tracking, data management and facilitate downstream statistical analysis.