Abstract
Regenerating gene IV (RegIV), a member of the regenerating gene family discovered in 2001, has been found to be involved in malignancy in several different organs including the stomach, colorectum, pancreas and prostate, but the overall expression profile of RegIV has not been reported. To learn more about RegIV, we evaluated its distribution by immunohistochemistry (IHC) in a total of 360 samples including 24 types of normal tissue, 40 benign and malignant lesions, and 18 neuroendocrine tumors. We found that in normal tissues, in addition to its relative specificity for the gastrointestinal tract, RegIV was detected in the adrenal gland and mammary gland. Among all the malignancies of various histological types under evaluation, RegIV was found mostly in adenocarcinomas. Studies on additional sets of colorectal tumor samples showed that RegIV expression was predominant in colorectal adenoma (87.5%) and peritumoral tissue (100%) but not in cancer tissue (30.8%). Among neuroendocrine tumors, RegIV had a relatively restricted expression to those of digestive system.
Keywords: Colorectal cancer, Expression profile, Neuroendocrine tumor, Regenerating gene IV (RegIV)
1. Introduction
The regenerating gene family, belonging to the C-type lectin superfamily, has widespread involvement in a number of cell and pathological processes including cell proliferation, inflammation, injury, diabetes and tumor formation (Dhar et al., 2004; Gurr et al., 2002; Sekikawa et al., 2005; Zhang et al., 2003b). Regenerating gene IV (RegIV), the most recently discovered member of the family, also referred to as regenerating protein-like protein (RELP), shares structural and functional features with other members of the family (Hartupee et al., 2001). Current data show that RegIV is detected as a differentially expressed gene in drug-resistant compared with drug-sensitive colorectal cancer cell lines (Violette et al., 2003). Like other members of the regenerating gene family, RegIV is over-expressed in inflammatory bowel disease and in tumors of the stomach, colorectum, prostate and pancreas (Gu et al., 2005; Oue et al., 2005; Takehara et al., 2006; Violette et al., 2003). In the normal stomach and colon, RegIV expression is weaker than in tumor tissues and shows two immunohistochemical staining patterns, mucin-like and perinuclear staining, which correspond to mucin secretory cells and differentiated neuroendocrine cells, respectively. In 1999, we constructed a complementary DNA (cDNA) library from extracts of colorectal normal mucosa or adenoma tissue from a single patient. RegIV was one of the genes screened out by suppression subtractive hybridization (SSH) as being differentially expressed in the normal mucosa and adenoma cDNA library (Zhang et al., 2003a). Little was known about how RegIV functions, except that it might be involved in the epithelium growth factor receptor (EGFR) signal pathway (Bishnupuri et al., 2006b). To investigate this further, we explored the expression profile of RegIV by immunohistochemistry (IHC) analysis in normal, benign and malignant human tissues, covering a much broader range than that in previous studies. We then investigated the relationship between RegIV expression and the clinical features of colorectal cancer. Since RegIV may be associated with neuroendocrine differentiation, we studied its distribution in various neuroendocrine tumors.
2. Materials and methods
2.1. Tissue samples
Specimens for IHC analysis were obtained at the time of surgery from the First Affiliated Hospital of Zhejiang University between 2000 and 2007. All samples were formalin-fixed and paraffin-embedded. RegIV distribution was detected by IHC analysis in 360 specimens from biopsies of various normal tissues, benign and malignant tissues, and neuroendocrine tumor tissues. Histological typing was confirmed morphologically. The study included 89 patients who had undergone surgical excision or removal of tumors by polypectomy for colorectal carcinoma (CRC) or adenoma. The adjacent normal tissues were retained in each specimen.
2.2. Antibodies
Both rabbit polyclonal and mouse monoclonal antibodies were raised against His-tagged recombinant RegIV protein produced in bacteria and purified with nickel resin (Novagen, Madison, WI, USA). The antibodies were purified by protein-A resin. Antibody titer was determined in a microtiter plate by indirect enzyme-linked immunosorbent assay (ELISA) using RegIV as the immobilized antigen. Specificity was evaluated by ELISA and immunoassay with antibody pre-incubated with RegIV recombinant protein. Mouse anti-chromogranin A (CgA) antibody was purchased from DakoCytomation (Glostrup, Denmark) as a neuroendocrine marker.
2.3. Immunohistochemical staining
Formalin-fixed, paraffin-embedded sections of all 360 tissues were subjected to the EnVision method using RegIV monoclonal antibody. RegIV expression was also detected using polyclonal antibodies in 89 CRCs with paired adjacent normal mucosa, including 24 with concurrent adenoma. The sections were deparaffinized and endogenous peroxidase activity was quenched by incubation for 30 min in 0.3% (v/v) hydrogen peroxide diluted in methanol. Antigen retrieval was then carried out using microwave pretreatment in citrate buffer for 20 min. Sections were then incubated with normal goat serum for 30 min to block non-specific binding sites, then treated consecutively with primary antibodies overnight at 4 °C. Negative controls were incubated in phosphate buffered saline (PBS) instead of the primary antibody. Finally, tissue sections were incubated with ready-to ready-to-use, peroxidase-conjugated goat anti-mouse/rabbit antibody (DakoCytomation, Glostrup, Denmark) for 30 min at room temperature, and developed with diaminobenzidine as chromogen. Sections were counterstained with hematoxylin.
2.4. Scoring method
Sections were scored according to the percentage of positive cells and the intensity of staining. The percentage of staining was scored as 0 (negative), 1 (≤30%), 2 (30%–70%), or 3 (≥70%); the intensity of staining was graded as 0 (negative), 1 (+), 2 (++), or 3 (+++). To obtain the total score, the staining score was added to intensity score. Any score below 2 was considered RegIV negative (−), 2–3 weak positive (+), 4 moderate positive (++), and 5–6 strong positive (+++).
2.5. Statistic analysis
The Chi-square test was used to analyze correlations between clinical parameters and the RegIV protein level, and to assess statistical differences between RegIV protein levels in normal versus adenoma versus carcinoma tissues.
3. Results
3.1. RegIV distribution in normal human tissues
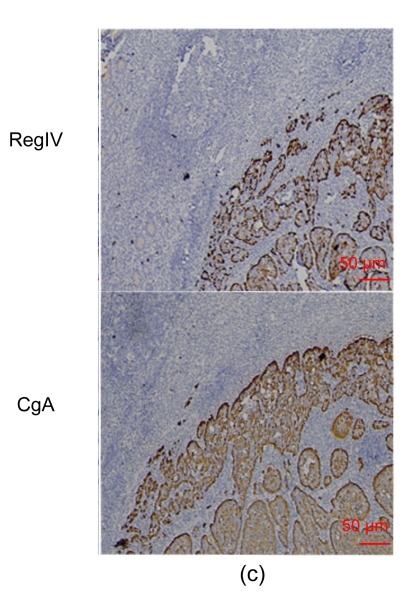
We detected RegIV expression in various normal human tissues, including the digestive system, adrenal gland and breast (Table 1 and Fig. 1). Extensive but not always strong RegIV staining was frequently found in the epithelium of the digestive system including the pancreas, stomach, small intestine, colorectum and appendix. The staining pattern was perinuclear in the crypt area and mucin-like in the gastrointestinal epithelial glands. Occasionally, RegIV positive staining was found in the myenteric nerve plexus. In adrenal tissue, strong RegIV immunostaining was detected in the adrenal medulla, with a clear demarcation from the cortex. Lobules of the mammary gland showed weak RegIV expression. We did not detect RegIV expression in the brain, lung, liver, kidney, bladder, prostate, ovary, cervix, endometrium, lymph node, tonsil, thyroid, salivary gland, pituitary gland, skin or spleen.
Table 1.
RegIV distribution in normal human tissues
| Tissue | Pos./total | Staining intensity/pattern |
| Respiratory system | ||
| Bronchus | 0/5 | |
| Lung | 0/4 | |
| Digestive system | ||
| Esophagus | 0/5 | |
| Stomach | 5/8 | 2 (+), 3 (+++); C/PN |
| Small intestine | 5/5 | 1 (++), 4 (+++); C/PN |
| Colorectum | 5/6 | 3 (+), 1 (++), 1 (+++); C/PN |
| Appendix | 2/3 | 1 (++), 1 (+++); C/PN |
| Liver | 0/6 | |
| Pancreas | 4/5 | 2 (+), 2 (++); C |
| Urinary system | ||
| Kidney | 0/7 | |
| Bladder | 0/4 | |
| Prostate | 0/4 | |
| Reproductive system | ||
| Ovary | 0/3 | |
| Cervix & endometrium | 0/5 | |
| Breast | 3/6 | 3 (+); C |
| Immune system | ||
| Lymph node | 0/7 | |
| Tonsil | 0/4 | |
| Exo/endocrine system | ||
| Thyroid | 0/5 | |
| Salivary gland | 0/1 | |
| Pituitary gland | 0/1 | |
| Adrenal gland | 1/3 | 1 (+++); C |
| Brain | 0/3 | |
| Skin | 0/6 | |
| Spleen | 0/4 |
Pos.: positive; C: cytoplasmic staining (sometimes mucin-like); PN: perinuclear staining; (+): weak positive; (++): moderate positive; (+++): strong positive
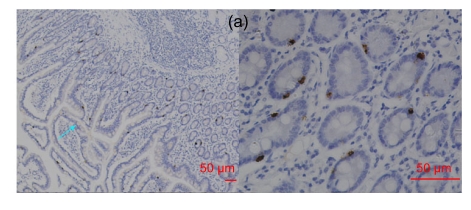
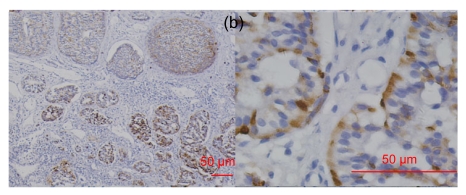
Fig. 1.
RegIV expression in normal human tissues
(a) In the small intestine, RegIV positive staining was strong in the perinuclear area and weak in goblet cells (blue arrow); (b) RegIV positive staining was detected in the cytoplasm of pancreatic acini cells; (c) Strong positive staining of RegIV was seen in the adrenal medulla, with clear demarcation with the cortex
3.2. RegIV distribution in benign and malignant tissues
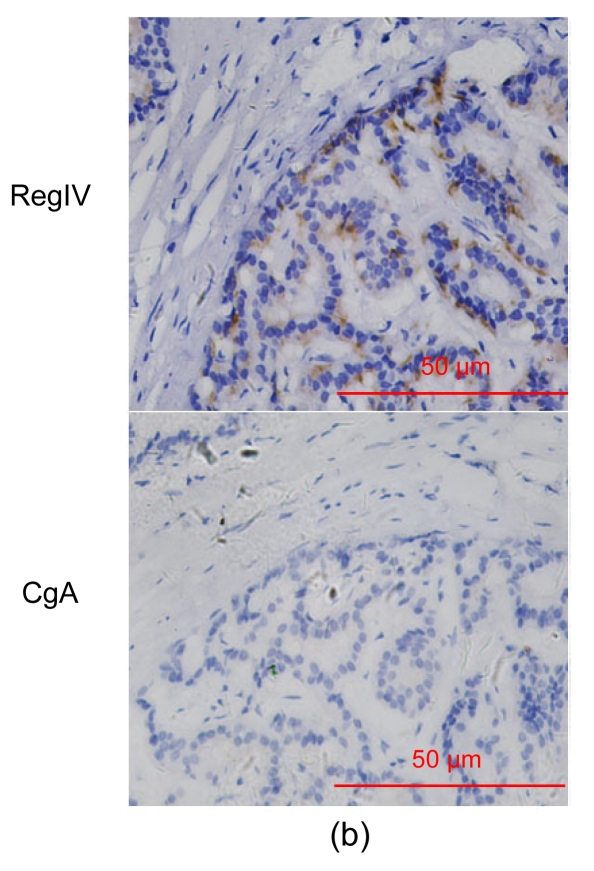
Forty types of tumoral tissues were subjected to IHC staining, including carcinomas, sarcomas and benign lesions (Table 2). RegIV expression was rarely detected in benign/malignant tissues other than those of the digestive system. Colorectal polyps showed frequent intensive RegIV expression. Some adenocarcinomas arising from the stomach, colorectum, pancreas, prostate or bladder were RegIV positive, among which the bladder was relatively weakly stained (Table 2, Fig. 2). All of the lung adenocarcinomas were negative. Furthermore, liver cholangiocarcinoma and infiltrating ductal carcinoma of the breast showed weak RegIV immunoreactivity. No positive signal for RegIV expression was found in neoplasia of other organs or histological types.
Table 2.
RegIV distribution in benign and malignant human tissues
| Tissue | Pos./total | Staining intensity/pattern |
| Carcinoma | ||
| Bronchioalveolar carcinoma | 0/5 | |
| Lung squamous cell carcinoma | 0/8 | |
| Esophagus squamous cell carcinoma | 0/5 | |
| Hepatocellular carcinoma | 0/9 | |
| Cholangiocarcinoma | 1/1 | (+); C |
| Renal cell carcinoma | 0/7 | |
| Transitional cell carcinoma | 0/5 | |
| Breast infiltrating ductal cancer | 1/4 | (+); C |
| Ovarian carcinoma | 0/3 | |
| Thyroid papillary carcinoma | 0/9 | |
| Thyroid follicular carcinoma | 0/3 | |
| Thyroid undifferentiated carcinoma | 0/3 | |
| Adenocarcinoma | ||
| Lung | 0/8 | |
| Gastric | 1/4 | (+++); C |
| Colorectal | 1/4 | (++); C |
| Appendix (SRCC) | 1/1 | (+++); C |
| Pancreas | 3/5 | 2 (++), 1 (+++); C |
| Bladder | 1/1 | (+); C |
| Prostate | 1/4 | (+++); C |
| Liver metastatic | 1/1 | (+++); C |
| Sarcomas | ||
| Fibrosarcoma | 0/2 | |
| Malignant fibrous histiocytoma | 0/2 | |
| Rhabdomyosarcoma | 0/2 | |
| Angiosarcoma | 0/2 | |
| Osteosarcoma | 0/2 | |
| Chondrosarcoma | 0/2 | |
| Others | ||
| Esthesioneuroblastoma | 0/4 | |
| Retinoblastoma | 0/5 | |
| Melanoma | 0/6 | |
| Seminoma | 0/6 | |
| Pancreatic cystic and solid tumors | 0/2 | |
| Mixed tumor of parotid gland | 0/2 | |
| Benign lesion | ||
| Lipoma | 0/6 | |
| Thyroid adenoma | 0/7 | |
| Thymoma | 0/5 | |
| Colorectal polyp | 10/11 | 2 (+), 3 (++), 5 (+++); M/PN |
| Glioma | 0/8 | |
| Junction nevus | 0/2 | |
| Intradermal nevus | 0/2 | |
| Teratoma | 0/5 |
Pos.: positive; SRCC: signet-ring cell carcinoma; C: cytoplasmic staining; PN: perinuclear staining; M: mucin-like staining
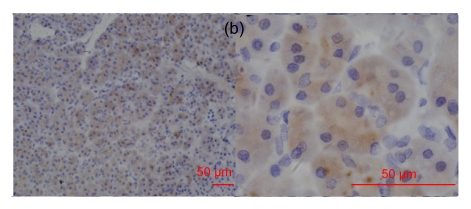
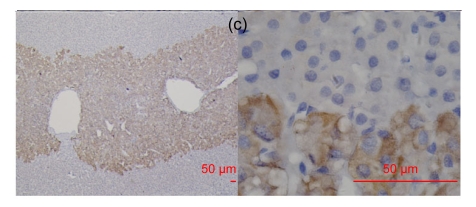
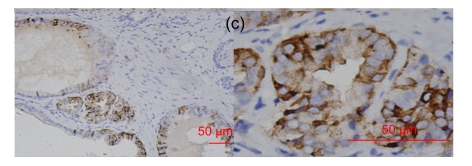
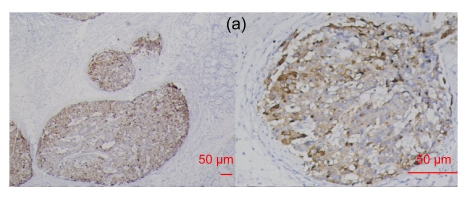
Fig. 2.
RegIV expression in adenocarcinomas
The histological types are ductal adenocarcinoma of the pancreas (a), colon signet-cell carcinoma (b), prostate adenocarcinoma (c) and liver metastatic adenocarcinoma (d), respectively
3.3. RegIV expression in colorectal tumors
As noted above, RegIV was relatively specific for the gastrointestinal tract including normal, benign and malignant tissues. To further our study, we investigated RegIV expression in an additional set of CRC samples. The correlation between RegIV expression and clinical features of CRC was also analyzed.
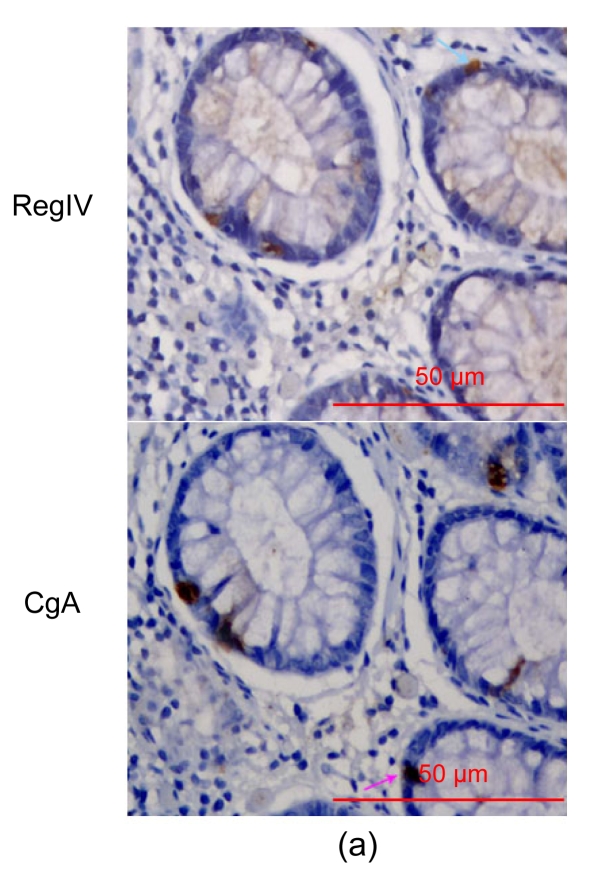
RegIV immunohistochemistry staining showed two patterns, mucin-like and perinuclear, both of which were seen in normal colorectal mucosa. By comparison with Alcian blue staining, we were able to demonstrate that 61.8% (55/89) of normal colorectal mucosa specimens showed positive mucin-like staining and 97.8% (87/89) showed positive perinuclear staining, which was usually weak and restricted to the crypt bases. In cancerous tissues, only positive mucin-like staining was observed (i.e., without positive perinuclear staining). The positivity in 89 cancer tissues was 33.7% (30/89). We analyzed the positivity separately in 65 CRCs without and 24 CRCs with concurrent adenoma. We found that the positivity was 30.8% in the 65 CRCs without concurrent adenoma, which was close to existing data (29%), and 41.6% in the 24 CRCs with concurrent adenoma (Tables 3 and 4) (Oue et al., 2007). The relationship between RegIV expression and clinicopathological features of CRCs was analyzed, and no correlation was found between RegIV expression and gender, age, tumor location, tumor size, lymph node metastasis or tumor stage. However, a significant association was found between RegIV staining and histological type of mucinous adenocarcinoma/signet-cell carcinoma (8/11, 72.7%; P=0.004), and poor differentiation (7/11, 63.6%; P=0.007) (Table 5). RegIV expression was found in 21 of 24 (87.5%) colorectal adenomas, all of which showed mucin-like staining, but only one of which also had perinuclear staining. The frequency of moderate/strong positivity of RegIV staining in normal, cancer and adenoma tissues was 19.1%, 14.6% and 70.8%, respectively. No correlation was found between RegIV staining and adenoma clinicopathological features (data not shown). Interestingly, among the four moderate/strong stained tumor sections of the 24 CRCs, three were serrated adenocarcinomas (Fig. 3b). As revealed by us and others, robust mucin-like RegIV staining could be detected in almost all of the pericancerous tissues and all of the periadenoma tissues (data not shown) (Oue et al., 2007). Perinuclear staining could also be found in perineoplastic tissues.
Table 3.
RegIV expression in CRC and corresponding normal and pericancerous mucosa (n=65)
| Staining pattern | Tissue | Positive case | Moderate/strong staining case |
| Mucin-like | Normal | 40/65 (61.5%) | 13/65 (20.0%) |
| Cancer | 20/65 (30.8%) | 9/65 (13.8%) | |
| Peri-cancer | 38/38 (100.0%) | 31/38 (81.6%) | |
|
| |||
| Perinuclear | Normal | 64/65 (98.5%) | |
| Cancer | 0/65 (0.0%) | ||
| Peri-cancer | 37/38 (97.4%) | ||
Table 4.
RegIV expression in colorectal adenoma and corresponding normal and carcinomatous mucosa (n=24)
| Staining pattern | Tissue | Positive case | Moderate/strong staining case |
| Mucin-like | Normal | 15/24 (62.5%) | 3/24 (12.5%) |
| Adenoma | 21/24 (87.5%) | 17/24 (70.8%) | |
| Cancer | 10/24 (41.6%) | 4/24 (16.7%) | |
| Peri-cancer | 18/19 (94.7%) | 11/19 (57.9%) | |
|
| |||
| Perinuclear | Normal | 23/24 (95.8%) | |
| Adenoma | 1/24 (4.2%) | ||
| Cancer | 1/24 (4.2%) | ||
| Peri-cancer | 19/19 (100.0%) | ||
Table 5.
Correlation of clinical features of CRC with RegIV expression
| Variable | No. of case # | RegIV expression (n=65) |
||
| Positive | Negative | P value | ||
| Gender | ||||
| Male | 33 | 10 (30.3%) | 23 (69.7%) | 0.934 |
| Female | 32 | 10 (31.3%) | 22 (68.7%) | |
| Age (year) | ||||
| ≤60 | 35 | 11 (31.4%) | 24 (68.6%) | 0.901 |
| >60 | 30 | 9 (30.0%) | 21 (70.0%) | |
| Tumor location | ||||
| Colon | 41 | 14 (34.1%) | 27 (65.9%) | 0.504 |
| Rectum | 23 | 6 (26.1%) | 17 (73.9%) | |
| Tumor size (cm) | ||||
| ≤5 | 19 | 3 (15.8%) | 16 (84.2%) | 0.072 |
| >5 | 41 | 16 (39.0%) | 25 (61.0%) | |
| Histological type | ||||
| Non-mucinous AC | 53 | 12 (22.6%) | 41 (77.4%) | 0.004 * |
| Mucinous AC/SRCC | 11 | 8 (72.7%) | 3 (27.3%) | |
| Differentiation | ||||
| Well/moderate | 53 | 13 (24.5%) | 40 (75.5%) | 0.029 * |
| Poor/undifferentiated | 11 | 7 (63.6%) | 4 (36.4%) | |
| Ulcer | ||||
| + | 43 | 11 (25.6%) | 32 (74.4%) | 0.162 |
| − | 21 | 9 (42.9%) | 12 (57.1%) | |
| Lymph node metastasis | ||||
| + | 32 | 13 (40.6%) | 19 (59.4%) | 0.147 |
| − | 32 | 7 (21.9%) | 25 (78.1%) | |
| Dukes stage | ||||
| Dukes A & B | 32 | 7 (21.9%) | 25 (78.1%) | 0.126 |
| Dukes C & D | 33 | 13 (39.4%) | 20 (60.6%) | |
Non-mucinous AC: non-mucinous adenocarcinoma; SRCC: signet-ring cell carcinoma
P<0.05, statistically significant
The number of cases is less than 65 in some items, because the information is unavailable
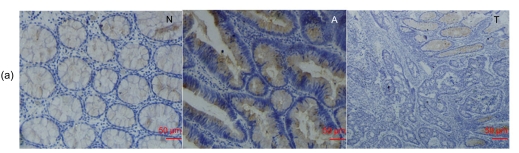
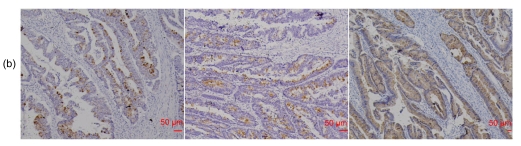
Fig. 3.
RegIV expression in colorectal adenoma
(a) RegIV expression in a CRC case with matched normal mucosa and adenoma. RegIV was weakly expressed in macroscopically normal mucosa (N); adenoma tissue (A) was intensively stained; in carcinoma (T), RegIV expression was negative, showing a sharp contrast with adjacent peri-cancer mucosa. (b) Three cases of serrated colorectal adenocarcinoma with moderate/strong RegIV staining
3.4. RegIV distribution in neuroendocrine tumors
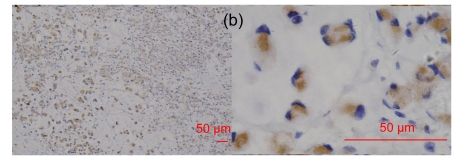
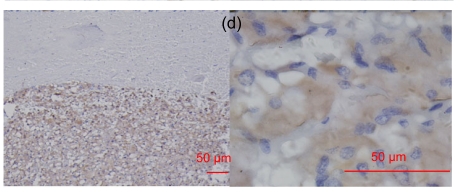
In the 18 neuroendocrine lesions, RegIV expression was positive in gastrointestinal neuroendocrine tumors from the stomach, colon, rectum and pancreas, but not in those from non-digestive sites except medullar thyroid carcinoma (usually weak) and pheochromocytoma (usually moderate/strong) (Fig. 4). CgA was examined for comparison (Table 6). Consecutive sections of normal colon stained for RegIV and CgA antibodies showed some cells stained for both antibodies and others for one or other of them. Only in some neuroendocrine tumor cases were RegIV and CgA both positive (Fig. 5).
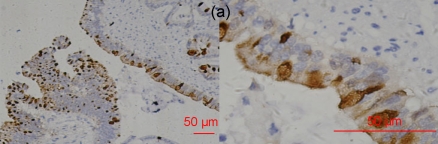
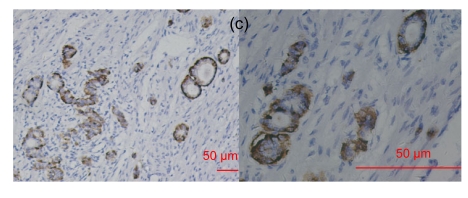
Fig. 4.
RegIV expression in neuroendocrine carcinomas
The intensive positive staining was restricted to the gastrointestinal tract and pheochromocytoma. (a) Gastric NEC; (b) Colonic carcinoid; (c) Appendix carcinoid; (d) Pheochromocytoma
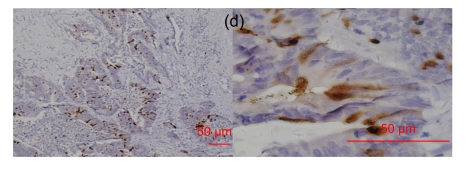
Fig. 5.
RegIV and CgA staining did not co-localize in normal colon and neuroendocrine carcinoma
(a) The blue and pink arrows showed their different positively-stained cells; (b) In the same neuroendocrine tumors, RegIV was positive, but CgA not; (c) In the same neuroendocrine tumors, RegIV and CgA were both positive
4. Discussion
In this study, by reference to 24 examples of normal tissues and 40 types of benign/malignant tissues, we have found that RegIV shows a restricted expression mainly in the digestive system but occasionally also at other sites. The prominent expression of RegIV in the gastrointestinal tract has been reported previously (Hartupee et al., 2001; Kamarainen et al., 2003). The adrenal was one of the extra-digestive system tissues where there was a restriction of RegIV staining to the medulla, and this corresponded with a majority of pheochromocytomas also staining positively and adrenocortical adenomas failing to stain.
In the study of cases where carcinoma was matched with normal tissue, RegIV expression in normal tissue was twice the level in carcinoma, but there was no significant difference between normal and tumoral tissues with regard to moderate/strong staining. Therefore, while RegIV expression in carcinomatous tissues is less frequent than that in normal tissues, it tends to be more intense when positive. Amongst adenocarcinomas, mucinous adenocarcinoma and signet-ring cell carcinoma showed enhanced RegIV expression. A similar finding was reported by Oue et al. (2005).
In the 24 CRCs with synchronous adenomas, RegIV expression was higher than in no-adenoma carcinomas. This may be the result of sampling error or a real biological tendency for CRC with concurrent adenoma to express RegIV. RegIV expression was also higher in adenomas than in normal or carcinomatous tissues (P<0.05), indicating that RegIV expression did not parallel the sequential normal-adenoma-carcinoma process. Also, strong positive RegIV staining in macroscopically uninvolved normal mucosa was always contrasted with adjacent tumor tissue, where RegIV staining was totally negative (Fig. 3a). A similar finding was published recently in relation to RegIV expression in the development of gastric cancer (Zheng et al., 2010). Currently, we cannot present a sound explanation for this phenomenon. Adenoma is a kind of precancerous lesion. Dieckgraefe’s group found that RegIV contributes to adenoma formation (Bishnupuri et al., 2006a). RegIV’s over-expression in precursors of neoplasia such as ulcerative colitis (Nanakin et al., 2007) and intestinal metaplasia (Kamarainen et al., 2003) has been demonstrated. Morphologically, so-called normal mucosa adjacent to adenoma or adjacent to adenocarcinoma is not completely normal, but has associated molecular alterations (Chen et al., 2004). While the significance of RegIV over-expression remains to be clarified, it may play an important role in the early (precancerous) stage of colorectal carcinogenesis. However, RegIV expression in CRCs was associated with poor differentiation, suggesting that RegIV may have multiple roles in colorectal carcinogenesis. In this study, we found that of the four cases from the 24-CRC-group with moderate/strong RegIV expression, three were serrated adenocarcinomas, one of which was characterized by large numbers of cells having perinuclear staining (Fig. 3b). Additional work on a larger sample of cases is required to confirm whether there is a specific association with serrated adenocarcinoma.
This is the first study reporting the distribution of RegIV in neuroendocrine tumors. In recent years, neuroendocrine tumors have become more prevalent, probably on account of improved detection methods (Yao et al., 2008). Diagnosing neuroendocrine tumors and their differentiation relies primarily on IHC. Neuron-specific enolase (NSE), CgA, and synaptophysin, perhaps the three most accepted and routinely used neuroendocrine markers, have their limits in diagnosing neuroendocrine tumors (Campbell et al., 2003; Kimura et al., 2000; Mani et al., 1994). More sensitive and specific markers are needed. In this study, we explored RegIV distribution in 18 types of neuroendocrine tumor. Comparison between CgA and RegIV staining in normal colon tissue revealed that positive cells did not correspond exactly (Fig. 5), as had been reported by Oue et al. (2005). Using IHC on different types of neuroendocrine tumor, we found that except for pheochromocytoma, RegIV expression was restricted to the digestive system, whereas CgA was not. Within the digestive system, positive cases of RegIV and CgA staining did not correspond well. Thus, we detected a high specificity of RegIV staining to gastrointestinal tract neuroendocrine tumors and especially carcinoid tumors. This could prove to have diagnostic importance if the results could be confirmed in a larger series.
In summary, we have demonstrated RegIV expression in various human normal, benign and malignant tissues. Being relatively specific for the digestive tract, RegIV plays an import role in digestive tract carcinogenesis, especially in adenocarcinoma or mucin-secretory tumor carcinogenesis. RegIV may widen the arsenal for the diagnosis of neuroendocrine tumors with a restricted expression to digestive tract neuroendocrine tumors and pheochromocytoma.
Footnotes
Project supported by the International Cooperation Program (No. 2007C24009), the Major Project of Natural Foundation of Zhejiang Province (No. D2080011), the Major Special Project of Science and Technology Found of Zhejiang Province (No. 2007C3020) and the Natural Science Foundation of Zhejiang Province, China (No. Y2090152)
References
- 1.Bishnupuri KS, Luo Q, Korzenik JR, Henderson JO, Houchen CW, Anant S, Dieckgraefe BK. Dysregulation of Reg gene expression occurs early in gastrointestinal tumorigenesis and regulates anti-apoptotic genes. Cancer Biol Ther. 2006;5(12):1714–1720. doi: 10.4161/cbt.5.12.3469. [DOI] [PubMed] [Google Scholar]
- 2.Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. RegIV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130(1):137–149. doi: 10.1053/j.gastro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Campbell LK, Thomas JR, Lamps LW, Smoller BR, Folpe AL. Protein gene product 9.5 (PGP 9.5) is not a specific marker of neural and nerve sheath tumors: an immunohistochemical study of 95 mesenchymal neoplasms. Mod Pathol. 2003;16(10):963–969. doi: 10.1097/01.MP.0000087088.88280.B0. [DOI] [PubMed] [Google Scholar]
- 4.Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, Moretto J, Mendes F, Smith AP, Bennington JL, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64(10):3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 5.Dhar DK, Udagawa J, Ishihara S, Otani H, Kinoshita Y, Takasawa S, Okamoto H, Kubota H, Fujii T, Tachibana M, et al. Expression of regenerating gene I in gastric adenocarcinomas: correlation with tumor differentiation status and patient survival. Cancer. 2004;100(6):1130–1136. doi: 10.1002/cncr.20097. [DOI] [PubMed] [Google Scholar]
- 6.Gu Z, Rubin MA, Yang Y, Deprimo SE, Zhao H, Horvath S, Brooks JD, Loda M, Reiter RE. RegIV: a promising marker of hormone refractory metastatic prostate cancer. Clin Cancer Res. 2005;11(6):2237–2243. doi: 10.1158/1078-0432.CCR-04-0356. [DOI] [PubMed] [Google Scholar]
- 7.Gurr W, Yavari R, Wen L, Shaw M, Mora C, Christa L, Sherwin RS. A Reg family protein is overexpressed in islets from a patient with new-onset type 1 diabetes and acts as T-cell autoantigen in NOD mice. Diabetes. 2002;51(2):339–346. doi: 10.2337/diabetes.51.2.339. [DOI] [PubMed] [Google Scholar]
- 8.Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: RegIV. Biochim Biophys Acta. 2001;1518(3):287–293. doi: 10.1016/s0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 9.Kamarainen M, Heiskala K, Knuutila S, Heiskala M, Winqvist O, Andersson LC. RELP, a novel human REG-like protein with up-regulated expression in inflammatory and metaplastic gastrointestinal mucosa. Am J Pathol. 2003;163(1):11–20. doi: 10.1016/S0002-9440(10)63625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura N, Pilichowska M, Okamoto H, Kimura I, Aunis D. Immunohistochemical expression of chromogranins A and B, prohormone convertases 2 and 3, and amidating enzyme in carcinoid tumors and pancreatic endocrine tumors. Mod Pathol. 2000;13(2):140–146. doi: 10.1038/modpathol.3880026. [DOI] [PubMed] [Google Scholar]
- 11.Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994;179(2):231–248. [PubMed] [Google Scholar]
- 12.Nanakin A, Fukui H, Fujii S, Sekikawa A, Kanda N, Hisatsune H, Seno H, Konda Y, Fujimori T, Chiba T. Expression of the RegIV gene in ulcerative colitis. Lab Invest. 2007;87(3):304–314. doi: 10.1038/labinvest.3700507. [DOI] [PubMed] [Google Scholar]
- 13.Oue N, Mitani Y, Aung PP, Sakakura C, Takeshima Y, Kaneko M, Noguchi T, Nakayama H, Yasui W. Expression and localization of RegIV in human neoplastic and non-neoplastic tissues: RegIV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol. 2005;207(2):185–198. doi: 10.1002/path.1827. [DOI] [PubMed] [Google Scholar]
- 14.Oue N, Kuniyasu H, Noguchi T, Sentani K, Ito M, Tanaka S, Setoyama T, Sakakura C, Natsugoe S, Yasui W. Serum concentration of RegIV in patients with colorectal cancer: overexpression and high serum levels of RegIV are associated with liver metastasis. Oncology. 2007;72(5-6):371–380. doi: 10.1159/000113147. [DOI] [PubMed] [Google Scholar]
- 15.Sekikawa A, Fukui H, Fujii S, Nanakin A, Kanda N, Uenoyama Y, Sawabu T, Hisatsune H, Kusaka T, Ueno S, et al. Possible role of Reg Iα protein in ulcerative colitis and colitic cancer. Gut. 2005;54(10):1437–1444. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takehara A, Eguchi H, Ohigashi H, Ishikawa O, Kasugai T, Hosokawa M, Katagiri T, Nakamura Y, Nakagawa H. Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci. 2006;97(11):1191–1197. doi: 10.1111/j.1349-7006.2006.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Violette S, Festor E, Pandrea-Vasile I, Mitchell V, Adida C, Dussaulx E, Lacorte JM, Chambaz J, Lacasa M, Lesuffleur T. RegIV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer. 2003;103(2):185–193. doi: 10.1002/ijc.10788. [DOI] [PubMed] [Google Scholar]
- 18.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35 825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YW, Lai MD, Lv BJ, Gu XM, Wang H, Zhu YM, Shao LN, Wang GF. Overexpression of RegIV in colorectal adenoma. Cancer Lett. 2003;200(1):69–76. doi: 10.1016/S0304-3835(03)00460-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YW, Ding LS, Lai MD. Reg gene family and human diseases. World J Gastroenterol. 2003;9(12):2635–2641. doi: 10.3748/wjg.v9.i12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng HC, Xu XY, Yu M, Takahashi H, Masuda S, Takano Y. The role of RegIV gene and its encoding product in gastric carcinogenesis. Hum Pathol. 2010;41(1):59–69. doi: 10.1016/j.humpath.2009.06.013. [DOI] [PubMed] [Google Scholar]