Abstract
Normal pregnancy is associated with significant hemodynamic changes in the cardiovascular system in order to meet the metabolic demands of mother and fetus. These changes include increased cardiac output, decreased vascular resistance, and vascular remodeling in the uterine and systemic circulation. Preeclampsia (PE) is a major complication of pregnancy characterized by proteinuria and hypertension. Several risk factors have been implicated in the pathogenesis of PE including genetic and dietary factors. Ca2+ is an essential dietary element and an important regulator of many cellular processes including vascular function. The importance of adequate dietary Ca2+ intake during pregnancy is supported by many studies. Pregnancy-associated changes in Ca2+ metabolism and plasma Ca2+ have been observed. During pregnancy, changes in intracellular free Ca2+ concentration ([Ca2+]i) have been described in red blood cells, platelets and immune cells. Also, during pregnancy, an increase in [Ca2+]i in endothelial cells (EC) stimulates the production of vasodilator substances such as nitric oxide and prostacyclin. Normal pregnancy is also associated with decreased vascular smooth muscle (VSM) [Ca2+]i and possibly the Ca2+-sensitization pathways of VSM contraction including protein kinase C, Rho-kinase, and mitogen-activated protein kinase. Ca2+-dependent matrix metalloproteinases could also promote extracellular matrix degradation and vascular remodeling during pregnancy. Disruption in the balance between dietary, plasma and vascular cell Ca2+ may be responsible for some of the manifestation of PE including procoagulation, decreased vasodilation, and increased vasoconstriction and vascular resistance. The potential benefits of Ca2+ supplements during pregnancy, and the use of modulators of vascular Ca2+ to reduce the manifestations of PE in susceptible women remain an important area for experimental and clinical research.
Keywords: calcium, pregnancy, hypertension, preeclampsia, endothelium, smooth muscle
INTRODUCTION
Physiological changes in normal pregnancy (Norm-Preg) adapt the cardiovascular system to the increased metabolic needs of the mother, and allow adequate delivery of oxygenated blood and nutrients to the fetus. Maternal cardiac output, plasma volume and renal blood flow increase during Norm-Preg (Baylis,1987; Sowers et al. 1990). The hypervolemia during the later half of Norm-Preg is related to increased plasma levels of estrogen and progesterone which induce the renin-angiotensin-aldosterone system, cause sodium retention and increase total body water. Other hormones, such as prolactin, placental lactogen, prostaglandins and growth hormone are increased during Norm-Preg and may contribute to water retention.
Norm-Preg is also associated with decreases in systemic and renal vascular resistance, and blood pressure (BP) (Baylis et al.1987; Sowers et al. 1990). The pregnancy-associated decrease in vascular resistance begins in week 5, with a nadir in weeks 20 to 32, and slowly increases after week 32 until full-term (Duvekot & Peeters, 1994). The pregnancy-associated changes in the systemic and renal hemodynamics are attributed to increased expression of nitric oxide synthase (NOS) and nitric oxide (NO) production by many cell types including renal and vascular cells (Deng et al. 1996, Sladek et al. 1997, Alexander et al. 1999, Nelson et al.2000, Abram et al.2001). Also, during Norm-Preg there is decreased pressor response and vascular reactivity to vasoconstrictor stimuli such as α-adrenergic agonists and angiotensin II (AngII) (Davidge & McLaughlin 1992; Khalil et al. 1998; Khalil & Granger, 2002).
In contrast to the decreased BP during Norm-Preg some women may have hypertension in pregnancy (HTN-Preg). HTN-Preg may present as: chronic HTN that predates pregnancy, preeclampsia (PE)-eclampsia, chronic HTN with superimposed PE, and gestational HTN, a nonproteinuric HTN-Preg (Khalil & Granger, 2002). In 5 to 7% of pregnancies in the US, 15% of pregnancies among African-Americans, women develop PE. PE manifests after the 20th week of pregnancy and is characterized by HTN and proteinuria. HTN is defined as systolic BP >140 or diastolic BP >90 mmHg in a woman known to be normotensive prior to pregnancy. Proteinuria is defined as urinary protein excretion ≥300 mg/24 hr. The clinical phase of PE is associated with enhanced pressor response to vasoconstrictors such as AngII and reduced renal plasma flow. Severe PE may be associated with oliguria, cerebral or visual disturbances, pulmonary edema, cyanosis, impaired liver function, thrombocytopenia, or intrauterine growth restriction. Another feature of PE is abnormal activation of the maternal coagulation cascade with an imbalance in production of the arachidonic acid metabolites thromboxane A2 (TXA2) and prostaglandin I2 (PGI2), favoring TXA2, as compared to Norm-Preg where an 8-fold increase in PGI2 production dominates a small increase in TXA2. Although PE-eclampsia represent a major cause of maternal and fetal morbidity and mortality, the triggering mechanisms of the hemodynamic and vascular changes have been elusive. PE remits dramatically postpartum after the delivery of the placenta, suggesting a role of the placenta. It has been suggested that reduction of uterine perfusion pressure (RUPP) in late pregnancy causes placental ischemia and initiates the release of biologically active factors such as vascular endothelial growth factor (VEGF) and inflammatory cytokines that cause the changes in maternal circulation and endothelial cell (EC) function observed in PE (Granger et al., 2002).
Due to the difficulty of performing mechanistic studies in pregnant women, several animal models of HTN-Preg have been developed. Surgical RUPP and induction of placental ischemia in late pregnant rats generates a phenotype mimicking many of the characteristics seen in human PE, including HTN, proteinuria and fetal growth restriction. The RUPP rat also demonstrates altered vascular functions as those observed in other animal models and in women with PE (Khalil & Granger, 2002; Gilbert et al., 2008).
PE has been linked to fetal and maternal genes, as well as mother's age, ethnic background, and health condition (Mackay et al. 2001). The incidence of PE may increase with dyslipidemia, hyperglycemia, chronic HTN, and with changes in dietary intake of proteins, vitamins and minerals such as Ca2+. Studies have examined dietary Ca2+ intake during the course of normal and complicated pregnancy. Possible correlations between dietary Ca2+ intake, plasma levels of Ca2+ and the incidence of PE have been examined. Also, the potential benefits of Ca2+ supplementation (Ca2+-Suppl) during pregnancy to reduce PE have been explored (Ramos et al. 2006); although the results have not been consistent. Also, while changes in vascular function play a major role in the control of vascular resistance and BP, little is known regarding the mechanisms of vascular contraction, relaxation and remodeling during pregnancy and PE. Intracellular free Ca2+ concentration ([Ca2+]i) plays a major role in regulating the functions of many cell types including blood and vascular cells. Increased [Ca2+]i in EC stimulates the production of vasodilator substances such as NO and PGI2. Also, VSM contraction is triggered by increases in [Ca2+]i. However, the regulation of dietary, plasma, and vascular tissue Ca2+ during Norm-Preg and the dysregulation of these mechanisms in the setting of the increased vascular resistance and BP in PE are not clearly understood.
The objective of this review is to examine reports in PubMed database and our research work to provide an insight on the inter-relationship between dietary, plasma and vascular cell Ca2+ during Norm-Preg and PE. We will first summarize the current understanding of the role of dietary Ca2+ and vitamin D, a co-factor in Ca2+ metabolism, during pregnancy. We will then describe the mechanisms of Ca2+ metabolism during pregnancy, and the changes in plasma Ca2+ levels in PE. We will then highlight the changes in [Ca2+]i in blood cells, platelets and immune cells during pregnancy. We will follow with description of pregnancy-associated changes in the Ca2+ regulatory mechanisms of EC and VSM cell function, and the extracellular matrix (ECM) of the vascular wall. The review will end with a perspective and clinical applications of the current knowledge on dietary, plasma and cellular Ca2+ during pregnancy, and the possible benefits of Ca2+-Suppl to reduce the incidence or manifestations of PE.
Dietary Ca2+ during Pregnancy
The Food and Nutrition Board of the National Academy of Science recommendation for Ca2+ intake is 1300mg/day for adolescents 9 to 18yr, and 1000 mg/day for adults 19-51yr (Bronner et al. 2006). The recommended dietary sources of Ca2+ are low fat dairy products (milk, cheese, yogurt) and certain green vegetables (broccoli, kale). Ca2+-Suppl are also available as chewable Ca2+ or in tablet form. The National Institutes of Health have defined the % Americans in different age groups that do not meet the recommended Ca2+ intake: 6-11yr 44% boys and 58% girls, 12-19 yr 64% boys and 87% girls, older than 20 yr 55% men and 78% of women. Among females, dietary Ca2+ is influenced not only by age, but also by ethnic background. Among girls 14-18 yr old the daily Ca2+ intake as % of the adequate dose is 58.1% by Caucasian, 48.2% by Hispanic and 44.9% by African-Americans (Storey et al. 2004). The daily Ca2+ intake can be further compromised by strict dietary regimens particularly among teen-agers. The nutritional status and dietary Ca2+ intake are of particular importance during pregnancy as they may affect the outcome of pregnancy and the postpartum condition of mother and fetus. The pregnant woman's body provides 50 to 300 mg/day Ca2+ for the developing fetal skeleton. Differences in total Ca2+ intake were observed between Caucasian (1556 mg/day) and African-American pregnant women (1421 mg/day) (Harville et al. 2004).
Most studies suggest that Ca2+-Suppl in the range of 375-2000 mg may be beneficial during pregnancy. Ca2+ deficiency may increase the risk of PE particularly among teenagers, and Ca2+-Suppl may reduce the incidence of PE. However, the beneficial effects of Ca2+-Suppl appear to vary depending on the mother's age and socioeconomic status, particularly in geographical locations where Ca2+ intake is low. The results could also depend on whether the subjects under study include normotensive women or women prone to PE, and whether the subjects have autoimmune disorders or under heparin therapy (Thomas & Weisman, 2006).
Randomized controlled clinical trails (RCCTs) have shown that Ca2+-Suppl is associated with reduced risk of HTN-Preg and PE (Table 1), and suggested that Ca2+-Suppl in a population with low Ca2+ intake is a safe, effective and inexpensive measure to reduce the risk of PE (Knight & Keith, 1992; Bucher et al. 1996; López-Jaramillo et al., 1997). Ca2+-suppl reduce the risk of PE and preterm birth particularly in nulliparous women (Crowther et al. 1999). Other studies were more skeptic regarding the effects of Ca2+-Suppl during pregnancy. The Ca2+ for PE Prevention (CPEP) trial has shown no significant effect of 2000 mg/day Ca2+-Suppl on BP or incidence of PE. Also, when the analysis was stratified according to patient's compliance, there was no effect on PE even among women who were most compliant with treatment (Levine et al. 1997). Other studies did not observe a lower risk of PE with greater dietary intake of Ca2+, vitamin C, D, or E, Mg2+, folate or elongated omega-3 fatty acids (Oken et al.2007). Further evaluation of these observations by the FDA have led to the suggestion that it is unlikely that consuming Ca2+-Suppl during pregnancy would reduce the risk of HTN-Preg or PE (Trumbo & Ellwood, 2007). Similarly, a World Health Organization RCCT of Ca2+-suppl among low Ca2+ intake pregnant women concluded that it did not prevent PE, although it did reduce PE severity, maternal morbidity and neonatal mortality (Villar et al. 2006).
Table 1.
Effects of dietary Ca2+-Suppl during pregnancy.
| Study | Study Design | Number of Women |
Ca2+-Suppl (mg/day) |
BP | Risk of Preeclampsia |
|---|---|---|---|---|---|
| Bucher, 1996 | meta-analysis of data from women at risk of PE in 14 RCCTs published in 1966-1994 |
2549 | 375-2000 | ↓ systolic and Diastolic BP |
↓ Compared with placebo, Ca2+-suppl reduced the odds for gestational HTN and PE |
| Knight, 1992 | RCCT in normotensive and HTN-Preg women |
50 | 1000 | ↓ Diastolic BP only in HTN-Preg women |
↓ BP-lowering effect of Ca2+-suppl is greater in women with PE |
| López-Jaramillo, 1997 | RCCT in Ecuador of 260 teens <17.5 yr; dietary Ca2+ ~600 mg/day |
260 | 2000 | ↓ systolic BP by 9.1 mmHg and diastolic BP by 6 mmHg |
↓ Decreased risk of PE. 3.2% developing PE in the treatment group and 15.5% in placebo group |
| Crowther, 1999 | Multi-center RCCT in Australia to assess the effect of Ca2+- Suppl daily from <24 wk to delivery |
456 nulliparous women with a singleton pregnancy |
1800 | not reported | ↓ Ca2+-suppl during pregnancy reduced the risk of PE and preterm birth in this nulliparous population |
| CPEP | Multi center RCCT in the United States |
4589 women of various ethnic and socioeconomic backgrounds with average Ca2+ intake 1100 mg/day |
2000 | _ | Ca2+-suppl did not reduce incidence of PE, or maternal and perinatal complications in nulliparous women, or prevent adverse pregnancy outcomes in adolescents or women with low base-line dietary Ca2+ intake or urinary Ca2+ excretion. |
| Oken, 2007 | 1718 | Evaluated food intake |
_ | _ | |
| WHO | Randomized trial of Ca2+-suppl among low Ca2+ intake pregnant women |
8325 | 1500 | _ | Ca2+-suppl did not prevent PE but did reduce its severity, maternal morbidity, and neonatal mortality |
| Hofmeyr, 2007 | Meta-analysis of 12 placebo- controlled RCCT |
15528 | 1000 | ↓ high BP | ↓ Decreased risk of PE |
↓ reduced risk
_ No change in risk
Interestingly, compiling data from 12 clinical trials, including some of the aforementioned studies, led to the conclusion that Ca2+-suppl may reduce the risk of HTN-Preg by 50%. The composite outcome maternal death or serious morbidity was also reduced. The effect was greatest in women at high risk of PE and those with low baseline Ca2+ intake (Hofmeyr, 2007).
The discrepancy in the effects of Ca2+-Suppl among various studies may be related to the study design. Significant heterogeneity was observed within the studies, with less effect of Ca2+-Suppl in the larger RCCTs (Hofmeyr et al., 2007). The discrepancy may also be related to the subjects' age. For example, studies that included large number of pregnant teen-agers, whose demand for Ca2+ exceeds that of pregnant adults, have shown that Ca2+-Suppl result in a significant decrease in BP and 12.4% decline in risk of PE (López-Jaramillo et al., 1997).
Experimental studies support beneficial effects of dietary Ca2+ during pregnancy. In pregnant ewes, restricted Ca2+ intake is associated with decreased plasma Ca2+ level and uterine blood flow, increased BP and elevated urinary protein; all symptoms similar to those of PE in women (Prada et al.1994). Also, low Ca2+ intake in Norm-Preg rats is associated with increased pressor effects of AngII (Aiko et al.,1992). Also, in both Norm-Preg and nonpregnant rats, a high Ca2+ diet (1.7%-2.1%) is associated with reduction in BP, and attenuated VSM reactivity in vitro. At the cellular level, the mechanism of attenuated reactivity appears to involve the EC-dependent NO-guanylate cyclase pathway (Ezimokhai & Osman, 1998).
Thus both clinical and experimental studies provided a tangible evidence for beneficial effect of Ca2+-Suppl during pregnancy. The Ca2+-Suppl may exert the strongest influence when dietary Ca2+ is low. If Ca2+ intake is adequate, no additional Ca2+-Suppl may be needed.
Vitamin D during pregnancy and PE
Vitamin D (cholecalciferol) is a major factor in normal Ca2+ absorption and metabolism. Cholecalciferol is formed by the skin during exposure to sunlight and UV irradiation, and also absorbed from ingested food. Absorbed cholecalciferol is converted in the liver to 25(OH)cholecalciferol or 25(OH)D, and further hydroxylated by 1α-hydoxylase in kidney to 1,25 dihydroxycholecalciferol or 1,25(OH)2D3, the most active form of the vitamin D group. 1,25(OH)2D3 is released in the blood stream and distributed to various target organs.
1,25(OH)2D3 induces genomic responses by diffusing in the cytoplasm and binding with strong affinity to vitamin D receptor, which promotes the transcription and translation of various proteins involved in bone formation (Fischer et al., 2007). 1,25(OH)2D3 is also involved in nongenomic responses that increase intracellular Ca2+. It stimulates Ca2+ transport from the intestine and renal tubules. Small concentrations promote bone calcification, while large concentrations augment bone resorption. Vitamin D receptors are also found in the placenta. 1,25(OH)2D3 levels are elevated during pregnancy due to increased activity of kidney 1α-hydoxylase and placental production (Perez-Lopez, 2007).
Adequate concentrations of 25(OH)D (>80 nmol/L) are needed for optimal health of the skeletal system, and also influence the cardiovascular system and BP. More than one-third of the US population suffer from vitamin D deficiency, defined as 25(OH)D <30 to 50 nmol/L. In Middle-Eastern countries vitamin D levels among women are very low. For example, in Morocco the prevalence of vitamin D deficiency (<30 ng/mL) is 91% (Allali et al., 2008).
Serum levels of 25(OH)D differ between men and women, Caucasian and African-Americans, and in winter compared to summer season (Burnand et al.1992). BP is known to increase in winter in the northern hemisphere where vitamin D synthesis in the skin is low. Results from the third National Health and Nutrition Examination Survey revealed an inverse association between circulating 25(OH)D and BP in Caucasian men and women. African-American men and women have significantly lower concentrations of 25(OH)D at all BP classifications than do Caucasian (Judd et al., 2008).
Studies in women supported an inverse relationship between estimated dietary intake of vitamin D and systolic BP (Sowers et al., 1985). In one study involving 148 women, women received 1200 mg Ca2+/day either alone or plus 800 IU vitamin D. Compared with Ca2+ alone, vitamin D plus Ca2+ resulted in 72% increase in serum 25(OH)D, 17% decrease in serum PTH, and 9.3% decrease in systolic BP (Pfeifer et al., 2001). Vitamin D requirements are increased during pregnancy, and the relation between vitamin D deficiency and the increased BP associated with HTN-Preg needs to be carefully examined. Studies have shown that circulating 1,25-(OH)2D3 levels in both maternal and umbilical cord compartments are lower in PE compared with Norm-Preg (Halhali et al., 2007).
Experimental studies examined the influence of vitamin D on the renin-angiotensin system. It was found that renin expression and plasma AngII production were increased in vitamin D receptor null mice, and were associated with HTN. Also, in wild-type mice, inhibition of 1,25(OH)2D3 synthesis led to increased renin expression, whereas 1,25(OH)2D3 injection caused renin suppression. Hence, 1,25(OH)2D3 is a negative regulator of the renin-angiotensin system and thereby plays a critical role in the regulation of BP (Li et al., 2002).
Plasma Ca2+
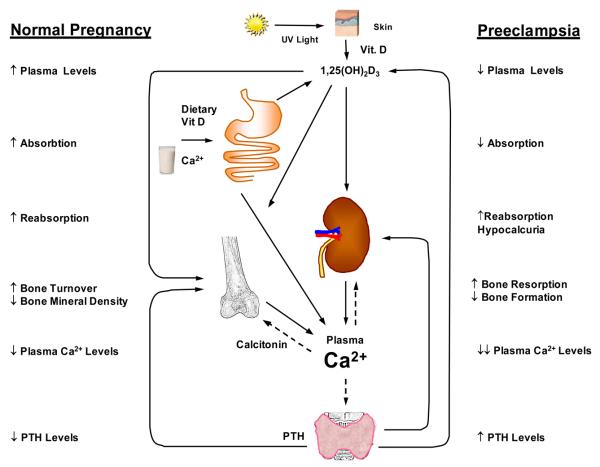
Ca2+ concentration in the extracellular fluid is strictly controlled and maintained at a normal serum level of 2.2-2.6 mmol/L; 40% bound to albumin, 10% in complex with citrate and the rest is ionized Ca2+ – the most important fraction. Three main factors affect the level of serum Ca2+ – 1,25(OH)2D3 (vitamin D3), parathyroid hormone (PTH) and calcitonin (Fig. 1).
Fig. 1.
Regulation of Plasma Ca2+. Vitamin D cholecalciferol is formed in the skin as a result of UV irradiation or absorbed from the gut, then hydroxylated to 25(OH)D in liver. Under the influence of parathyroid hormone (PTH), 25(OH)D is further hydroxylated in kidney to 1,25(OH)2D3, the most active form of vitamin D. PTH stimulates 1,25(OH)2D3 formation. 1,25(OH)2D3 stimulates Ca2+ transport from intestine, reabsorption in renal tubules and release from bone. Calcitonin inhibits Ca2+ mobilization from bone.
PTH increases serum Ca2+ levels by inducing bone resorption, intestinal transport, and renal tubular reabsorption. PTH also stimulates the formation of 1,25(OH)2D3, which in turn stimulates Ca2+ transport from the intestine and renal tubules, and in large quantities augments bone resorption. Calcitonin, a peptide hormone secreted by the parafollicular cells in the thyroid gland, tends to decrease plasma Ca2+ level mainly by inhibiting osteoclasts and suppressing Ca2+ mobilization from bone. Calcitonin also has modest effect on kidney.
Other hormones also influence Ca2+ homeostasis. Adrenal steroids decrease osteoblast function and bone formation, and increase osteoclast number and activity. Glucocorticoids decrease intestinal Ca2+ absorption and renal Ca2+ reabsorption, and augment renal excretion. Excess glucocorticoids cause osteoporosis. Thyroid hormones may cause hypercalcemia and hypercalciuria. Growth hormone facilitates intestinal absorption and renal excretion of Ca2+.
During pregnancy Ca2+ is transferred to the fetus via the placenta. The higher Ca2+ requirement during pregnancy requires physiologic adaptation of the Ca2+ homeostatic mechanisms including intestinal absorption, urinary excretion, and maternal bone Ca2+ turnover. These mechanisms are partly influenced by the placenta estrogen, progesterone, and human chorionic gonadotropin. Intestinal Ca2+ absorption rises during the first weeks of pregnancy, and reaches a maximum in the last trimester. In contrast, urinary excretion of Ca2+ decreases during pregnancy. Biochemical markers of bone formation (serum osteocalcin and bone alkaline phosphatase) and bone degradation (N-telopeptides of type I collagen) increase during pregnancy. Ca2+ absorption is positively associated with serum 1,25(OH)2D3 and PTH (Cross et al.,1995). Plasma 1,25(OH)2D3 levels increase two-fold early in pregnancy due to high placental 1α-hydroxylase activity, remain high until delivery and decline to normal values during lactation. The levels of estrogen, prolactin and placental lactogen, which are involved in Ca2+ absorption, increase at the same time (Ritchie et al.,1998; Vargas Zapata et al., 2004).
During Norm-Preg, the increased levels of 1,25(OH)2D3 and the facilitation of intestinal Ca2+ absorption results in hypercalcemia, which causes PTH levels to decrease (Seely et al., 1992). Also, in response to hypercalcemia, calcitonin is secreted and suppresses Ca2+ reabsorption in the renal tubules and results in hypercalciuria. Calcitonin also counteracts the bone resorptive effects of 1,25(OH)2D3, and thus protects the maternal skeleton (Whitehead et al., 1981). In some studies, urinary Ca2+ conservation in pregnancy was associated with an increase in serum PTH. However, in women with habitually high Ca2+ intake, the association between reduced urinary Ca2+ and increased PTH was not evident. When comparing the different stages of pregnancy, PTH levels were low in early pregnancy, declined toward mid-term, but increased in late pregnancy (Davis et al., 1988; Seki et al., 1991). Other studies demonstrated that during pregnancy PTH levels were not different from the levels of nonpregnant controls (Gillette et al.,1982; Saggese et al., 1991). In pregnant rats, PTH levels were higher than in nonpregnant rats (Bourdeau et al.1990). Opposite results have shown that PTH and Ca2+ levels were lower in Norm-Preg than nonpregnant rats (Gonen et al., 2005).
Estrogen regulates bone metabolism, prohibiting bone loss and inhibiting stimulatory effect of cytokines on osteoclasts. Therefore, the high gestational estrogen is predicted to protect maternal bone. However, during pregnancy maternal bone mineral density (BMD) decreases, suggesting bone loss (Fukuoka & Haruna, 2003). In rats, Norm-Preg increases BMD, whereas lactation decreases it. Changes in PTH levels contribute to mineralization and demineralization of the skeleton during pregnancy and lactation, respectively (Gonen et al., 2005).
Studies have demonstrated lower levels of 1,25(OH)2D3 and PTH in HTN-Preg and suggested that alterations in Ca2+ regulatory hormones could contribute to the development of PE, and the symptom of hypocalciuria (August et al.,1992). Other studies suggest that in PE the low levels of 1,25(OH)2D3 cause a decrease in intestinal Ca2+ absorption and serum ionized Ca2+. The lower ionized Ca2+ causes an increase in PTH levels, which stimulate Ca2+ loss from bones, and tubular reabsorbtion of Ca2+ from kidney leading to hypocalciuria (Seely et al., 1992). Therefore, urinary Ca2+ can be an early marker for PE (Sanchez-Ramos et al., 1991). Hypocalciuria could be a compensatory mechanism for the increased total vascular resistance and decreased renal blood flow in PE (Szmidt-Adjide et al., 2006).
Studies have shown that the plasma Ca2+ levels go down a little during Norm-Preg and markedly decrease in women with PE (Seely et al., 1992; Kisters et al., 2000; Sukonpan & Phupong, 2005) (Table 2). However, in one study serum Ca2+ was reduced and fractional renal excretion of Ca2+ (FECa) increased in Norm-Preg compared to nonpregnant controls. In PE, serum Ca2+ was not different from that in Norm-Preg group, but FECa was lower. PTH was slightly lower during Norm-Preg than after delivery, but did not deviate from the nonpregnant group. In PE, PTH did not deviate from the levels in Norm-Preg. Also, calcitonin was the same in the third trimester of pregnancy in both groups. It was concluded that both Norm-Preg and PE are accompanied by considerable alterations in Ca2+ metabolism, that PTH and calcitonin in both groups are mainly unchanged from nonpregnant level, and that the increase and decrease in renal Ca2+ excretion in Norm-Preg and PE, respectively, may be attributed to changes in kidney function (Pedersen et al., 1984). Other studies have also demonstrated no difference in serum ionized Ca2+ between Norm-Preg and HTN-Preg subjects (Richards et al., 1984).
Table 2.
Plasma and vascular cell Ca2+ levels in healthy non-pregnant, normal pregnant, and preeclamptic women.
| Parameter | Non-pregnant | Normal pregnant | Preeclampsia | References |
|---|---|---|---|---|
|
Plasma Volume (ml/m2) Serum [Ca2+] (mmol/L) (mg/dL) 1,25(OH)2D3 (pg/ml) PTH (ng/L) |
~2000 2.2-2.6 2.22-2.54 8.9-10.2 50.4 +/− 17.3 24.8+/−9.0 |
2279 +/− 325 1.26+/−0.01 2.2 +/− 0.1 9.7 +/− 0.7 50+/−9 15.4+/−1.3 |
1790 +/−332 1.20+/−0.01 1.96 +/− 0.5 9.0 +/− 0.4 43+/−9 29.9+/−4.3 |
Silver et al., 1998 Seely et al.,1992 Kisters et al., 2000 Sukonpan et al., 2005 Halhali et al.,2007 Davis et al., 1988 Seely et al.,1992 |
|
24 h Urinary Ca2+ Excretion (mg/day) |
50-250 | 189.24±57.06 | 71.20±22.95 | Ray et al.,1999 |
|
Red blood cells Count (1012/L) Cytosolic [Ca2+] (mEq/L) Membrane Ca2+ (μmol/g) |
4.7-6.1 0.0149±0.0023 0.83±0.16 |
4.23±0.40 0.015±0.001 0.77±0.13 |
4.14±0.41 0.033±0.010 1.23±0.36 |
Ceyhan et al.,2006 Sowers et al., 1989 Kosch et al., 2000 |
|
White blood cells Count (109/L) Absolute neutrophil count Cytosolic [Ca2+] (nmol/L) |
4.5-10 2500-7000 47±9 |
10.864±3.350 7,498.6±2,354.0 94±3 |
11.261 ±0.528 9,410±3,066.9 121±7 |
Ceyhan et al.,2006 Lurie et al., 1998 Hojo, 1999 |
|
Platelets Count (109/L) Main platelet volume (fL) Cytosolic [Ca2+] (nmol/L) |
150-450 6.3-9.5 112.3±2.9 |
220±79 9.45±1.11 120.1±5.7 |
227±71 9.18±1.52 163.6±8.8 |
Ceyhan et al., 2006 Ceyhan et al., 2006 Kilby et al., 1990 |
|
Endothelial Cells Cytosolic [Ca2+] sVCAM-1 (marker of activation) (ng/ml) vWf (marker of damage) (IU/dL) |
+ 558±20 90±47 |
++ 1,159.8±340 123±24 |
+++ 2,269±426 206.9±40.6 |
Haller et al.,1997 Bouchlariotou, 2008 Bouchlariotou, 2008 |
|
Cultured rat VSM treated with human sera [Ca2+]i Basal (nmol/L) AngII (10−8M) % Increase over baseline |
158±14 236±16% |
135±15 370±14% |
160±14 65±12% |
Green et al., 2000 Green et al., 2000 |
Experimental studies have shown higher total plasma Ca2+ in Norm-Preg than non-pregnant rats. Both the plasma total and ionized Ca2+ concentration were lower in L-NAME treated rat model of HTN-Preg compared to Norm-Preg rats (Ebose et al., 2007) (Table 3).
Table 3.
Plasma and vascular cell Ca2+ levels in non-pregnant, normal pregnant and hypertensive pregnant rat
| Non pregnant | Norm-Preg | HTN-Preg | Reference | |
|---|---|---|---|---|
| Serum Ca2+ (mg/dL) | 10.14±0.09 | 10.67±−0.18 | 10.29±0.08 | Ebose et al., 2007 |
| Endothelial cell [Ca2+]i (nM) | 84 ±4 | 138 ± 5 | TBD | Gokina et al, 2006 |
| VSM [Ca2+]i (nM) - Basal - PHE (10−5M) [Ca2+]i (nM) Initial Maintained |
86 ±6 417 ± 11 183 ± 8 |
63 ± 5 414±13 149 ±8 |
109 ± 8 429±19 234 ± 11 |
Murphy et al., 2001 Murphy et al., 2001 |
Regulation of [Ca2+]i
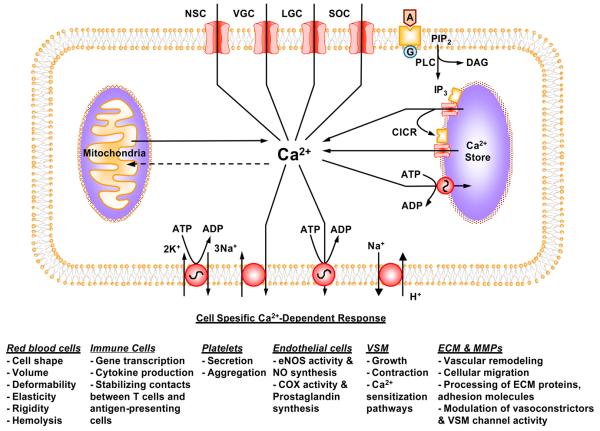
Ca2+ is a major regulator of the function of various vascular cells. [Ca2+]i homeostasis is tightly regulated by Ca2+ mobilization and Ca2+ extrusion mechanisms (Fig. 2). The Ca2+ mobilization mechanisms include Ca2+ release from the intracellular stores, and Ca2+ influx from the extracellualr space through voltage-gated, ligand-gated and store-operated Ca2+ channels. Excess Ca2+ is either taken up by Ca-ATPase in the intracellular store membrane, or extruded via plasmalemmal Ca-ATPase and Na+/Ca2+-exchanger. Extremely high cellular Ca2+ may be taken up by the mitochondria. The Ca2+ regulatory pathways are slightly different in various vascular cells in order to adapt for the specific cell function (Fig. 2).
Fig. 2.
Regulation of [Ca2+]i and Ca2+-dependent cellular response. During cell activation, Ca2+ is released from the intracellular stores through IP3-sensitive Ca2+ channels and the ryanodine-sensitive Ca2+-induced Ca2+ release mechanism. Extracellular Ca2+ enters the cell through voltage-gated, ligand-gated, store-operated, and nonspecific channels. The increased [Ca2+]i triggers specific responses in red blood cells, platelets, immune cells, endothelial cells, and VSM cells. When the cell stimulus is removed, [Ca2+]i returns back to normal level via the Ca2+-ATPase pump and Na/Ca exchanger. Also, the Na/K-pump and Na/H exchanger could affect the membrane potential and intracellular pH and further modify the Ca2+ response. Extremely high levels of [Ca2+]i are taken up by the mitochondria.
Ca2+ in Red blood cells
Blood volume, as determined by measuring red blood cell volume and plasma volume, is reduced in PE (Silver et al.,1998). Although plasma Ca2+ levels may be low in PE, the erythrocytes [Ca2+]i and membrane Ca2+ content are increased, suggesting altered membrane ion transport (Table 2). The highest BP measurements were recorded in PE women with extremely high erythrocytes [Ca2+]i (Kisters et al.,2000). The increased erythrocytes [Ca2+]i in PE may be caused by an increase in PTH (Sowers et al., 1989; Kosch et al., 2000).
Ca2+ uptake by red blood cell membranes is reduced by ~50% in PE compared with Norm-Preg, suggesting that the membrane Ca-ATPase activity is diminished in PE (Nardulli et al.1994, Ray et al.1999; Teppa-Garrán et al., 2004; Oviedo et al., 2006). Ca-ATPase activity in the myometrium and placental membranes is also 50% lower in PE than Norm-Preg women, suggesting an endogenous inhibitor of Ca-ATPase in PE (Javed et al., 2000; Carrera et al.,2003). The reduced Ca-ATPase activity of the red cell membranes from PE women was not associated with reduced number of Ca-ATPase molecules or a defective enzyme, but rather with a high level of lipid peroxides (Oviedo et al., 2006). A positive correlation was also found between systolic and diastolic BP and plasma levels of malondialdehyde, a lipid peroxidation product (Kaur et al., 2008). The role of lipid peroxidation and Ca2+ in PE is supported by reports that increased lipid peroxides inhibit Na,K-ATPase and Ca-ATPase (Carrera et al., 2003). These changes may occur in other cell types of PE women, and lead to an increase in [Ca2+]i, and some of the symptoms of PE. Lipid peroxidation is partly responsible for the endothelial damage associated with PE (Kaur et al., 2008). Also, a dysfunctional Ca-ATPase could result in increased [Ca2+]i and vasoconstriction in VSM. Increased lipid peroxides in the placental tissue is also caused by the uteroplacental hypofusion, a triggering event in the pathogenesis of PE (Carrera et al., 2003).
Ca2+ in Platelets
In platelets, basal [Ca2+]i is ~100 nmol/L and is regulated by the intracellular Ca2+ stores, membrane channels and Ca-ATPase pump (Kilby et al., 1993). Platelets are activated in early Norm-Preg, but the degree of their activation is controlled by yet unknown buffering factors that maintain hemostasis and prevent further platelet activation. For example, in Norm-Preg the platelets show refractoriness to activation by IL-1 and TNF-α. This refractoriness is lost or diminished in PE, and platelet activation by cytokines occurs weeks to months before the clinical appearance of PE. Failure to control the platelet activation during pregnancy leads to platelet adhesion, aggregation, and release of TXA2, which in turn, cause vasoconstriction, further aggregation, and progressive damage to EC (Bar et al.,2004). Also, in PE women during the third trimester, the platelets show more extensive activation, indicated by the increased expression of P-selectin (CD62P), CD63, plasma β-thromboglobulin, and platelet EC adhesion molecule 1 (PECAM-1) (Janes et al.1995; Konijnenberg et al.1997).
Platelet aggregation and secretion are associated with a rise in [Ca2+]i. Several studies suggest that platelet [Ca2+]i is increased in Norm-Preg women in the third trimester, and highly increased in PE (Haller et al., 1989; Kilby et al.1993) (Table 2). Other studies have shown no significant difference in basal platelet [Ca2+]i between Norm-Preg and PE women (Barr et al.1989, Zemel et. al 1990). While some studies showed exaggerated response of platelet [Ca2+]i to arginine vasopressin early in pregnancy (Zemel et. al 1990), other studies did not show similar results (van der Post et al.1993, Kyle et al.1995).
In Norm-Preg increased platelet [Ca2+]i stimulates TXA2 formation and lowers level of cAMP which further increase [Ca2+]i and enhance platelet aggregation (Sheu et al. 2002). TXA2 also acts as a vasoconstrictor (Herrera et al., 2006). An increase in platelet [Ca2+]i in PE, if present, may explain the increased secretion and aggregability seen in this condition. Studies have shown that platelets from PE women require higher concentrations of PGE1 to inhibit aggregation than those from women with nonproteinuric gestational hypertension indicating increased platelet activity in PE women (Torres et al.1996). PGE2, a vasodilator produced by ECs, inhibits platelet aggregation (Herrera et al., 2006). In contrast, platelets from women with HTN have high basal secretory levels of TXA2 (Hawkins et al.1993). Also, the sensitivity and [Ca2+]I response of platelets to AngII is increased in PE compared to Norm-Preg women (Haller et al.1989). Platelets cGMP is reduced in PE compared to Norm-Preg, indicating decreased amount/action of NO (Teran et al. 2004). In contrast with the inconsistent data in human PE, experimental studies suggest that the platelet resting [Ca2+]i is higher in L-NAME-treated rat model of HTN-Preg compared with Norm-Preg rats (Ebose et al., 2007) (Table 3).
Ca2+ in Immune Cells
There is compelling evidence of peripheral immune cell activation in PE. Leukocyte activation may be secondary to EC activation by circulating syncytiotrophoblast microvillous membranes (STBM) shed from the PE placenta. EC co-cultured with STBM cause significant activation of granulocytes and monocytes as indicated by increased [Ca2+]i, decreased pHi and release of ROS. Lymphocytes respond mainly with an increase in ROS (von Dadelszen et al. 1999). Serum from PE patients increases ICAM-1 expression on EC surface, which in turn binds and activates leukocytes. Also, expression of integrin counter receptors on leukocytes is increased in PE and Norm-Preg compared with the nonpregnant state, and the expression decreases significantly postpartum (Haller et al.1997). Polymorphonuclear neutrophils (PMN) are the cells most affected in PE, with changes in expression of surface markers and release of granule enzymes. PE is associated with changes in L-selectin on PMN, monocytes and T cells when compared with Norm-Preg. These changes include increased nuclear translocation of NF-κB and levels of IL-6 (Luppi et al.2006). PMN generate superoxide anion (O2−•), and O2−• generation is reduced in Norm-Preg compared with nonpregnant controls, but is increased in PE and lead to EC dysfunction (Lee et al.2003, Aly et al.2004). Also, during PE, PMN produce TXA2 and TNF-α in response to oxidative stress (Vaughan et al.2005).
[Ca2+]i is a key regulator in immune cells. Activation of PMN is associated with rapid elevation in [Ca2+]I due to Ca2+ release from the intracellular stores and Ca2+ entry through plasma membrane channels such as store-operated Ca2+ channels (Steinckwich et al.2007). A correlation between ROS production and Ca2+ entry suggest that endogenous ROS reinforce Ca2+ signaling by positive feedback (Giambelluca & Gende, 2008). PMN are essential for the phagocytosis and killing of pathogenic bacteria, an action that depends on the production of ROS by the phagocyte NADPH oxidase and on the release of proteases. The same effector mechanisms could cause tissue damage in the case of inappropriate PMN activation. Maternal plasma levels of elastase may serve as a marker of PMN activation (Gupta et al., 2006). PMN [Ca2+]i is higher in PE, compared with healthy pregnant and non-pregnant women (von Dadelszen et al., 1999). Intracellular Ca2+ flux is an early step in the signaling cascade that bridges stimulation of selectin and chemokine receptors to activation of adhesive and motile functions (Schaff et al., 2008). The importance of Ca2+ is confirmed by the finding that Ca2+ channel blockers suppress cytokine-induced PMN activation (Shima et al., 2008). Membrane depolarization accompanies activation of the phagocyte NADPH oxidase. NADPH oxidase regulates PMN membrane potential and Ca2+ influx via its electrogenic activity and as a result of generation of ROS (Tintinger et al., 2007).
In PE, changes in [Ca2+]i occur in other immune cells (von Dadelszen et al., 1999). Peripheral blood monocytes from pregnant women secrete low levels of vasoactive prostanoids and respond to PGI2, PGE2 and TXA2 in a manner similar to that of nonpregnant women. The cells from women with HTN-Preg demonstrate increased reactivity, exaggerated rise in TXA2 secretion, and prostacyclin (PGI2) to a lesser extent (Hawkins et al.1993).
In lymphocytes isolated from decidua of PE women, secretion of Th2-type cytokines IL-6 and IL-10 is decreased, while the Th1-type cytokine IFN-γ is increased (Wilczyński et al.2002). Elevation of [Ca2+]i is one of the triggering signals for T-cell activation by antigen. Stimulation of the T cell receptor, activates IP3 and ryanodine receptors and Ca2+ release from the intracellular stores, and depletion of the stores triggers Ca2+ influx through store-operated Ca2+ (CRAC) channels. The amplitude and dynamics of the Ca2+ signal are controlled by several mechanisms, including K+ channels, membrane potential, plasma membrane Ca-ATPase, and mitochondria that buffer Ca2+ and prevent inactivation of CRAC channels (Lewis et al., 2001).
NF-κB is a key regulator of immune responses. Phosphatidylinositol 3-kinase (PI3K) and its downstream target Akt, as well as Ca2+/calmodulin-dependent protein kinase and calcineurin are implicated in NF-κB regulation (Chen et al.,2002). Lymphocyte basal [Ca2+]i is higher in PE than Norm-Preg women (Table 2). Exposure of lymphocytes to low extracellular Ca2+ results in an increase in [Ca2+]I, and may serve as a marker of PE (Hojo et al., 1999).
Ca2+ in Endothelial Cells (ECs)
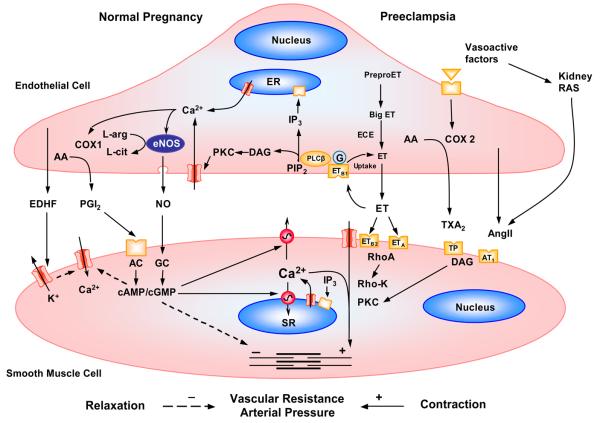
[Ca2+]i is a major regulator of EC function. Both hemodynamic shear stress and Ca2+ mobilizing agonists elicit a rise in EC [Ca2+]i (Fig. 3). Also, Ca2+ entry triggered by intracellular Ca2+ store depletion regulate many EC functions. ECs release relaxing factors such as NO, PGI2, and hyperpolarizing factor as well as contracting factors such as TXA2, endothelin (ET-1), AngII and PAF. A rise of [Ca2+]i activates Ca2+-dependent enzymes such as NOS and phospholipase A2, leading to the production and release of NO and PGI2. [Ca2+]i also plays a role in cytokine production, induction of adhesion molecules, disassembly of EC junctions and increased EC permeability. Also, in ECs induction of NF-κB by various stimuli requires [Ca2+]i for proper signal transduction (Nilius & Droogmans, 2001).
Fig. 3.
Regulation of endothelial and VSM [Ca2+]i during Norm-Preg and preeclampsia. Norm-Preg is associated with increased expression and Ca2+-dependent activation of endothelial eNOS and COX, leading to increased NO and PGI2 production. NO and PGI2 act on VSM, causing an increase in cGMP and cAMP, which activate Ca2+ extrusion and VSM relaxation mechanisms. Increased EDHF during pregnancy causes VSM hyperpolarization, and inhibition of Ca2+ entry through voltage-gated channels. In preeclampsia, a decreased NO bioavailability mainly due to increased ROS would cause reduction of VSM relaxation. Also, the release of bioactive factors such as cytokines from the placenta and other sources causes an increase in the release of endothelium-derived contracting factors such as ET-1 and TXA2 and AngII which in turn cause an increase in VSM [Ca2+]i and the Ca2+-sensitization pathways such as PKC and Rho-kinase, leading to enhanced vasoconstriction, increased vascular resistance and BP.
EC [Ca2+]i depends on Ca2+ entry through membrane channels, Ca2+ pumps, and Ca2+ release from intracellular stores. Ca2+ entry occurs through nonselective cation channels including: 1) Agonist or receptor-activated cation channels involving phospholipase C, but the downstream second messengers are unclear; 2) Amiloride-sensitive channels may regulate cation fluxes across the blood-brain barrier; 3) Redox nonselective cation channels activated by oxidative stress and equally permeable for Na+, K+ and also Ca2+; 4) Cyclic nucleotide-gated channels. Nonselective cation channels are mechanosensitive and increase [Ca2+]i in response to shear stress or increased flow. Mechanical forces also affects K+ and Cl− channels causing changes in membrane potential and VGCCs. Store-operated or capacitative Ca2+ entry is controlled by the filling degree of intracellular Ca2+ stores and is a major pathway for Ca2+ influx during agonist stimulation. These channels are much more selective for Ca2+ and some belong to the trp gene family, which encodes the transient receptor potential channels (TRPCs). Two highly Ca2+-selective agonist-activated channels are activated by ATP and bradykinin. Ca2+ also enters ECs via reverse-mode Na+/Ca2+ exchanger, where reduction of the Na+ gradient allows more Ca2+ entry and increases [Ca2+]i (Nilius & Droogmans, 2001).
In ECs, NO synthases (NOS) convert L-arginine to L-citrulline with the release of NO. Endothelial eNOS is a constitutive Ca2+-calmodulin dependent isoform. Increased blood flow causes ECs to synthesize NO. Also, receptor ligands such as Ach activate ECs and stimulate Ca2+ release from the intracellular stores. Depletion of intracellular Ca2+ stores signals an influx of extracellular Ca2+ which activates the eNOS associated with the plasma membrane and promotes NO production. This is supported by reports that inhibiting extracellular Ca2+ influx eliminate histamine-stimulated NO production in ECs (Lantoine et al., 1998). NO diffuses to the VSM layer where it activates guanylyl cyclase, producing cGMP, and induces relaxation.
NO plays a major role in gestational vasodialation (Noris et al, 2004). The pregnancy-associated increases in NOS expression/activity are likely due to increased levels of sex hormones. In female guinea pigs, near-term pregnancy and treatment with estradiol (but not progesterone) cause 4-fold increase in Ca2+-dependent NOS activity in the uterine artery. Also, pregnancy-associated increase in NOS activity in the cerebellum is inhibited by the estrogen receptor antagonist tamoxifen. Both pregnancy and estradiol treatment cause increases in eNOS and nNOS mRNA in skeletal muscle, suggesting that the increased NOS activity result from enzyme induction (Weiner et al., 1994).
PE is associated with EC dysfunction. Women with overt PE show increases in circulating levels of markers of EC injury such as cellular fibronectin, clotting factor VII, von Willebrand factor and factor VIII-related antigen (Anumba et al., 1999; Granger et al., 2001; Khalil & Granger, 2002). EC injury is also manifested in one of the most characteristic morphologic lesions of PE, glomerular endotheliosis. In PE, the ischemic placenta and other maternal tissues may release a factor(s) into the systemic circulation that cause EC activation and subsequently dysfunction. EC injury sets in motion a cascade of coagulation, vasoconstriction, and intravascular fluid redistribution that result in the clinical syndrome of PE. Some of the factors released during PE include cytokines such as TNF-α, IL-6, IL-1α and IL-1β, growth factors such as VEGF, lipoproteins or lipid peroxides, ROS, hypoxia inducible factors (HIFs) and neurokinin-B (Vince et al.1995; Davidge et al.1996; Benyo et al.1997; Conrad et al.1998; Lamarca et al., 2007). TNFα destabilizes electron flow in mitochondria, resulting in release of oxidizing free radicals and formation of lipid peroxides, which in turn cause endothelial cell damage (Conrad & Benyo, 1997). Elevated levels of asymmetric dimethyl arginine (ADMA), endogenous inhibitor of eNOS, precede the development of PE (Savvidou et al.2003). Also, STBM shed from the placenta contain factors which could cause EC damage in PE (von Dadelszen et al.1999, Smárason et al.1993). Studies on myometrial and subcutaneous resistance have shown loss of vasodilator effect of Ach, but not substance P in microvessels of PE compared to Norm-Preg women (Wimalasundera et al., 2005).
VEGF may play in the endothelial cell changes associated with PE. Polymorphisms of the VEGF gene (36C/T) are associated with development of PE (Papazoglou et al., 2004; Shim et al., 2007). VEGF may regulate the baseline NO production from ECs. Activation of VEGF, EGF and PDGF receptor tyrosine kinase induces upregulation of eNOS. VEGF also causes a biphasic increase of [Ca2+]i and stimulates production of NO in human and rabbit ECs (Van der zee et al. 1997). In resting ECs, eNOS is localized in EC caveolae, small invaginations of the plasma membrane that are abundant in the transmembrane protein caveolin. Caveolin-1 tightly binds to a motif in the oxygenase domain of eNOS and maintains it in an inactive state within caveolae. VEGF receptor stimulation leads to phosphorylation of caveolin-1. Consequently, eNOS dissociates from caveolin-1 and is activated by intracellular Ca2+ which is also increased by VEGF (Mukherjee et al.2006). Plasma levels of VEGF and placenta growth factor (PGF) are reduced in severe PE (Livingston et al.2000). Also, the placental expression and secretion of a naturally occurring circulating VEGF antagonist, soluble fms-like tyrosine kinase 1 (sFlt1), is increased in PE (Koga et al.2003, Tsatsaris et al.2003; Karumanchi & Epstein, 2007). When tested in rats, sFlt1 alone induces PE-like phenotype. sFlt1 binds circulating VEGF and prevents its interaction with its EC receptors and thereby lead to EC dysfunction in PE (Kendall & Thomas, 1993, Levine et al.2004). Some studies have shown that VEGF is increased before the clinical onset and further elevated during the vasoconstriction state of PE. The hyperdynamic circulation during the latent phase of PE causes vascular shear stress, which in turn increases the levels of circulating VEGF. Because VEGF normally acts as a vasodilator, its increase may represent an unsuccessful vascular rescue response (Bosio et al.2001).
Some studies have shown that NO levels during PE are equal or higher than in Norm-Preg (Boccardo et al.1996, Anumba et al.1999, Rowe et al.2003). The basal EC [Ca2+]i is higher in PE compared to Norm-Preg (Haller et al.1997). In contrast, histamine-stimulated Ca2+ entry is reduced in fetal umbilical vein ECs from PE compared with Norm-Preg women. Basal and histamine-stimulated cGMP levels were elevated in PE compared with normal cells, implying increased NO production in PE. These data suggest an altered cation membrane permeability and activity of eNOS-sGC pathway in fetal ECs from women with PE (Steinert et al., 2002).
In cultured ECs, exposure to serum from PE women results in increased expression of NOS and NO, release of PDGF, fibronectin which promotes platelet aggregation, increased secretion of ET-1, induction of oxygen radicals, and inhibition of PGI2 production (Taylor et al.1991a,b, Baker et al.1995, 1996; Davidge et al.1995, Gallery et al.1995). The release of some of these cellular mediators is partly due to increased ECs [Ca2+]i upon exposure to PE serum. A high [Ca2+]i in ECs of PE women could be involved in the upregulation of NOS and may produce excess NO which under oxidative stress (also present in PE) produce excess peroxynitrate, a highly reactive intermediate that cause nitration of protein tyrosine residues and cellular oxidative damage. ROS can also activate NF-κB and induce the expression of adhesion molecules in ECs (Saraswathi et al.2004). Some studies suggest that expression of NOS is not different in placentae of normal and PE women (Conrad & Davis, 1995), while other studies suggest that human placental NOS activity is significantly reduced in PE (Brenneckeet al, 1997).
In PE, ECs can activate leukocytes and vice versa (Mantovani & Dejana,1989). Serum from PE patients contain factors that increase EC permeability and ICAM-1 expression on ECs. Thus, increased adhesiveness of leukocytes in PE is likely due to changes on ECs rather than alterations on leukocyte surface. The increased adhesion could contribute to the enhanced coagulation and diminished fibrinolysis seen in PE (Haller et al.1997). In addition to [Ca2+]i, the effect of PE serum on ECs could also involve PKC-α and -ε (Haller et al.1998).
In pregnant rats, both endogenous NO and cGMP production are increased. Also, NO deficiency by administering NOS inhibitors in pregnant rats produces a syndrome of HTN and proteinuria that resemble PE (Yallampalli & Garfield.1993, Khalil et al., 1998; Crews et al.1999). Supplementation with L-arginine reduces the HTN in the RUPP rat model of HTN-Preg (Alexander et al.2004). Studies have suggested that RUPP and the ensuing placental ischemia/hypoxia cause an increase in the release of cytokines into the maternal circulation, which in turn lead to the changes in vascular function and BP [Vince et al., 1995; Conrad et al., 1997; Williams et al., 1998; Khalil & Granger, 2002; Stennett & Khalil, 2006]. In support of the cytokine hypothesis, the plasma levels of TNF-α are elevated in women with PE [Conrad et al., 1997; Williams et al., 1998]. Immune cells may represent another source of the elevated circulating levels of TNF-α in PE [Benyo et al., 2001]. We have shown that infusion of TNF-α or IL-6 in Norm-Preg rats, to elevate its plasma level to levels similar to those in PE, is associated with increased BP and systemic vasoconstriction [Davis et al., 2002; Orshal & Khalil, 2004]. Also, treatment of vascular segments from pregnant rats with TNF-α or IL-6 reduces vascular relaxation and NO production [Giardina et al., 2002; Orshal & Khalil, 2004].
Ca2+ in Vascular Smooth Muscle (VSM)
[Ca2+]i is a major determinant of vascular tone. Ca2+ flux to and from the VSM cytosol is regulated by Ca2+ release from the intracellular stores, Ca2+ entry from the extracellular space, and Ca2+ extrusion mechanisms (Fig. 3). Under resting conditions, the opening of K+ channels permits the exit of K+ from the cell which hyperpolarizes VSM cell membrane and closes VGCCs. The Na/K-ATPase pump also produces membrane hyperpolarization and further counter-regulates VGCCs. During VSM activation, increased [Ca2+]i is initiated by IP3-induced Ca2+ release and ryanodine-sensitive Ca2+-induced Ca2+ release mechanisms. Vasoconstrictor agonists also stimulate Ca2+ entry through ligand-gated and VGCCs. Relaxing factors released from ECs act on VSM and inhibit phospholipase C, open K+ channels or stimulate [Ca2+]i extrusion, and thereby decrease [Ca2+]i. EC dysfunction is associated with decreased release of relaxing factors, and decreased VSM Ca2+ extrusion. EC dysfunction also causes an increase in VSM contracting factors, which stimulate Ca2+ mobilization from the intracellular stores and Ca2+ entry from the extracellular space. Contraction of VSM to hypoxia is also mediated by accumulation of [Ca2+]i (Ramón de Berrazueta et al.1999). Ca2+ binds calmodulin to form a complex which activates myosin light chain (MLC) kinase, causes MLC phosphorylation, initiates actin-myosin interaction and produces VSM contraction.
Some studies suggest that vasoconstrictors play a role in PE (Mizutani & Tomoda, 1996). Other studies on women myometrial and subcutaneous resistance vessels have shown that microvessel reactivity to KCl solution, PHE and AngII were not increased in PE compared with Norm-Preg, and suggested that this is an unlikely mechanism of the increased peripheral vascular resistance in PE (Wimalasundera et al, 2005).
Circulating autoantibodies that activate the AngII AT1 receptor are also considered a factor in PE. AT1 autoantibodies (AT-1AA) rise at the time the symptoms of PE develop and subside within 6 weeks after delivery. AT-1AA were purified and characterized as a fraction of IgG antibodies. AT-1AA activity may promote Ca2+ signaling and VSM contraction. Also, AT-1AA induced mobilization of intracellular Ca2+ initiate a signaling cascade that culminate in activation of NF-κB and activator protein-1 and subsequently tissue factor expression (Dechend et al.2000; Thway et al., 2004). Other studies have postulated the influence of placental proteinases on angiotensin activity and VSM contraction. It has been suggested that the decreased pressor responsiveness to AngII in Norm-Preg is caused by increased inactivation of AngII by angiotensinase in serum and placenta. Angiotensinase activity increases with advancing gestation in Norm-Preg sera, but the enzyme activity is lower in severe PE sera leading to decreased degradation and increased sensitivity to AngII (Mizutani & Tomoda, 1996). Enhanced VSM responses in PE could also be related to increased ROS production. ROS cause increases in [Ca2+]i by activating VGCCs. ROS can also increase [Ca2+]i by promoting IP3-induced Ca2+ release from the intracellular stores and by inhibiting plasma membrane Ca2+-ATPase activity (Jin et al., 2004).
Although studies have suggested that the increased vascular reactivity associated with HTN-Preg may be partly due to enhanced activity of VGCCs in VSM (Ebeigbe et al, 1987), the results have not been consistent. Studies compared the effects of sera from Norm-Preg, PE, pregnant women with chronic HTN, non-pregnant women with HTN, and age-matched non-pregnant normotensive women on [Ca2+]i in cultured rat aortic VSM cells. After 4 hr incubation period with serum, basal [Ca2+]i was not significantly altered. However, compared with Norm-Preg sera, PE sera markedly reduced hormonally induced Ca2+ transients (Green et al.2000).
Our studies in arterial VSM cells of rats have shown that basal and agonist-stimulated [Ca2+]i are reduced in Norm-Preg compared with virgin rats, but significantly elevated in pregnant rats treated with L-NAME (Murphy et al., 2001) (Table 3). In VSM cells incubated in a Ca2+-containing solution, PHE causes an initial peak followed by maintained increase in [Ca2+]i. The PHE- and caffeine-induced cell contraction and transient increase in [Ca2+]i in Ca2+-free solution are not significantly different between Norm-Preg and virgin rats nontreated or treated with L-NAME, suggesting that the pregnancy-associated changes in VSM contraction are not due to changes in Ca2+ uptake to or Ca2+ release from the intracellular Ca2+ stores. In contrast, PHE-induced maintained [Ca2+]i in Ca2+-containing medium, a measure of Ca2+ entry from the extracellular space, is reduced in Norm-Preg rats, but enhanced in L-NAME-treated pregnant rats (Murphy et al.2001). Also, KCl-induced VSM cell contraction and [Ca2+]i are reduced in Norm-Preg compared with virgin rats but enhanced in L-NAME-treated pregnant rats, providing evidence that Ca2+ entry through VGCCs is reduced during Norm-Preg but enhanced in L-NAME-treated pregnant rats. The cause of the reduced Ca2+ entry into VSM in Norm-Preg rats and its enhancement in L-NAME-treated pregnant rats could be related to possible changes in permeability or number of Ca2+ channels. [Ca2+]i may also play a role in the changes in VSM contraction in RUPP rats. In renal arterial VSM, enhanced mechanisms of Ca2+ entry, rather than release from the intracellular Ca2+ stores, may be a contributing factor to the increased cell contraction and [Ca2+]i in response to AngII and KCl in RUPP rats as compared to Norm-Preg rats (Murphy et al., 2003).
VSM Ca2+-Sensitization pathways
In addition to Ca2+/calmodulin-dependent MLC kinase, protein kinase C (PKC), Rho-kinase, and mitogen-activated protein kinase (MAPK) contribute to VSM contraction. Activation of PKC by phorbol esters causes sustained contraction of VSM with no detectable change in [Ca2+]i, suggesting an increase in Ca2+ sensitivity of the contractile proteins. Increased VSM PKC expression/activity has been identified in HTN (Horowitz et al.1996; Salamanca & Khalil, 2005). VSM PKC activity may play a role in the vascular changes observed in Norm-Preg and PE. The Ca2+ sensitivity of VSM contractile elements is increased in women with PE (Vanwijk et al.2002). PE patients develop IgG autoantibody to VSM AT1R, and PKC may play a role in the changes in AT1R-mediated signaling associated with PE. In cultured neonatal rat cardiomyocytes immunoglobulin from PE women enhances AT1R-mediated chronotropic response, whereas IgG from control subjects has no effect. Treatment of cardiomyocytes with the PKC inhibitor calphostin C prevented the stimulatory effect of IgG from PE women on AT1R-mediated chronotropic response. Confocal microscopy showed colocalization of IgG from PE women and AT1R antibody in VSM cells. These studies suggest that PE patients develop stimulatory autoantibodies against AT1R, a process that appears to be mediated via PKC [Wallukat et al., 1999].
Studies on uterine artery from pregnant sheep and the aorta of late pregnant rats have shown that the decreased vascular contraction during Norm-Preg is associated with decreased PKC activity [Magness et al., 1991; Kanashiro et al., 2000]. Also, the expression and subcellular redistribution of Ca2+-dependent α-PKC and Ca2+-independent δ- and ζ-PKC are reduced in aortic VSM from pregnant compared to nonpregnant rats [Kanashiro et al., 1999; 2000]. BP is greater in late pregnant rats treated with the NOS inhibitor L-NAME compared with Norm-Preg or virgin rats. Also, PHE-induced contraction is greater in aortas from L-NAME treated pregnant rats compared to Norm-Preg or virgin rats [Khalil et al., 1998; Crews et al., 1999]. Additionally, vascular PKC activity and the expression and subcellular redistribution of α- and δ-PKC were enhanced in L-NAME treated pregnant rats compared with Norm-Preg rats. Thus an increase in the expression/activity of α- and δ-PKC isoforms may play a role in the increased vasoconstriction and vascular resistance associated with HTN-Preg [Kanashiro et al., 1999; 2000]. RUPP and the ensuing placental ischemia/hypoxia in late pregnancy may induce the release of cytokines into the maternal circulation, which in turn lead to the generalized changes in vascular function associated with HTN-Preg [Stennett & Khalil, 2006; Lamarca et al., 2007]. Cytokines likely increase the expression/activity of VSM PKC, leading to increased myofilament force sensitivity to [Ca2+]i and enhanced VSM contraction.
Rho protein is a family of small GTP binding proteins that are involved in many cellular functions including cell proliferation, migration, cytoskeletal reorganization and contraction. Rho-kinase is activated by GTP binding and is inactivated by hydrolyzing GTP to GDP, and this process is influenced by various stimuli including growth factors and vasoactive substances. Two Rho-kinase isoforms encoded by two different genes have been identified, ROCK1 and ROCK2. Targeting Rho protein or its downstream effector protein Rho-kinase may have therapeutic potential in HTN (Hu et al. 2006). Agonists such as noradrenaline, ET-1 and TXA2 bind to G-protein-coupled receptors and produce contraction by increasing both [Ca2+]i and the Ca2+ sensitivity of the contractile apparatus. The increased Ca2+ sensitivity of VSM results from inhibition of MLC phosphatase activity leading to increased MLC phosphorylation and tension at a constant [Ca2+]i. A major component of the Ca2+-sensitizing effect of vasoconstrictors is ascribed to RhoA-mediated ROCK activation (Loirand et al. 2006; Somlyo et al., 2003). ROCK inhibitors (Y-27632, fasudil) normalize arterial BP in animal models of HTN indicating the importance of ROCK signaling in the vascular hyperreactivity associated with HTN (Uehata et al.1997). The importance of Rho in PE is supported by a study comparing subcutaneous resistance arteries from PE, Norm-Preg and nonpregnant women, and showing that Ca2+ sensitivity of the contractile elements is increased in women with PE (VanWijk et al., 2002). Other studies have shown that mRNA expression of Rho-kinase is downregulated in umbilical arteries of PE women (Friel, 2006). Stimulation of AT1R may induce upregulation of RhoA/ROCK activity in hypertensive rats (Kataoka et al., 2002). An increase in AngII activity during PE may activate ROCK and promote vasoconstriction.
MAPKs are ubiquitously expressed serine/threonine protein kinases that play an important role in cell differentiation, growth, apoptosis, and the regulation of transcription factors and gene expression. MAPKs include extracellular signal-regulated kinase 1/2 (ERK1/2), big MAPK1, c-Jun N-terminal kinase and p38. The ERK family mediates growth factor-stimulated cell differentiation and growth, while JNK and p38 mediate inflammatory cytokine- and stress-induced apoptosis and stress-responsive gene expression. ROS and MAPK promote vascular remodeling in many pathological conditions and may be involved in PE. ROS are increased in PE, and MAPKs are sensitive to oxidative stress (Kyaw et al., 2004). Tyrosine kinase and MAPK activities have been identified in differentiated VSM, suggesting a role in VSM contraction [Khalil et al., 1995]. In differentiated VSM cells, agonist-induced activation and generation of diacyglycerol at the surface membrane promotes the translocation of ε-PKC from the cytosol to surface membrane. The activated ε-PKC promotes the translocation of both MAPK kinase (MEK) and MAPK to the plasmalemma, where they form a complex. PKC induces phosphorylation and activation of MEK, which in turn phosphorylates MAPK at both Thr and Tyr residues (Adam et al., 1992; Khalil et al., 1995). Tyrosine phosphorylation of MAPK targets it to the contractile myofilaments, where it phosphorylates the actin-binding protein caldesmon (D'Angelo et al., 1999; Hedges et al., 2000). The phosphorylation of caldesmon reverses its inhibition of actin-mediated MgATPase activity, increases the actin-myosin crossbridge cycling and enhances VSM contraction [Khalil et al., 1995]. A decrease in VSM PKC expression during Norm-Preg and potential increase in HTN-Preg likely change the MAPK-caldesmon phosphorylation pathway and thereby VSM contraction.
Ca2+ and Extracellular matrix (ECM)
Norm-Preg is associated with significant remodeling of the cytoskeleton and ECM in blood vessels of the uterine and systemic circulation. Matrix metalloproteinases (MMPs) are a family of structurally related, zinc-containing enzymes that promote proteolysis, degrade ECM proteins and play a role in vascular remodeling and cell migration. An essential step in MMP-induced proteolysis is conversion of the zymogen into an active proteinase by various mechanisms including proteolysis, allosteric interactions, oxidative modification and Ca2+. Increased extracellular Ca2+ concentration ([Ca2+]e) may promote degradation of ECM components by increasing MMP activity. In mouse fibroblasts, increasing [Ca2+]e induces release and activation of MMP-2 and -9. The MMPs release could be dependent on store-operated Ca2+ channels and the NO/cGMP/PKG pathway negatively regulates this Ca2+ entry pathway (Huang et al., 2006). Also, PAF induces MMP-9 expression in human vascular ECs through Ca2+- or PI3K-dependent signaling pathway (Ko et al., 2005).
Studies suggest that plasma levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 are higher in HTN-Preg than Norm-Preg women (Merchant et al.2004; Tayebjee et al.2004). Other studies have shown that the levels of MMP-2 and -9 in decidua cells are not elevated in PE (Huisman et al., 2004). Also, while the levels of MMP-9 may be higher in decidual cells and adjacent interstitial trophoblasts in placenta of PE versus Norm-Preg women, the levels of MMP-2, TIMP-1 and TIMP-2 were similar (Lockwood et al.2008). MMP-9 mRNA expression and protein levels in trophoblasts may be even reduced in PE (Qiao et al.2005). Also, treatment of trophoblast-like cell culture with PE serum causes reduction of MMP-2 (Mahameed et al.2005). A decreased MMP-2 content/activity during PE would reduce collagen breakdown in the umbilical cord artery. The accumulation of collagen with simultaneous reduction in elastin content in the umbilical cord artery may reduce elasticity of arterial wall and decrease blood flow to the fetus in women with PE (Galewska et al.2003). Also, a low decidual MMP-1 expression in PE may inhibit endovascular invasion by cytotrophoblasts, and partly explain the relative failure of trophoblasts to invade maternal decidual blood vessels (Gallery et al.1999).
MMPs may also affect vascular reactivity. MMP-2 cleaves big ET-1 to ET-1, which could activate ETA receptors and produce vasoconstriction, and therefore MMP-2 inhibitors may evoke vasorelaxation (Fernandez-Patron et al.2000). However, MMP-2 can process big ET-1 at the gly-leu bond to ET1–32 which activates endothelin B (ETB) receptor-NO signaling pathway and promote vasodilation (Davison et al.2004). Relaxin upregulates vascular gelatinase activity during pregnancy, and thereby contributes to renal vasodilation, hyperfiltration, and reduced myogenic reactivity of renal arteries through activation of the EC ETB receptor-NO pathway (Jeyabalan et al.2003). The source of the increased plasma MMPs is unclear, but the placenta is considered a potential source. MMP-2 and -9 are increased in the placenta of diabetic rats at mid-gestation (Pustovrh et al.2007). Also, VEGF has been shown to induce the expression and promote the secretion of MMPs from ECs (Narumiya et al.2001).
We have shown that MMP-2 and -9 cause relaxation of rat aorta and inferior vena cava (Chew et al., 2004; Raffetto et al.2007). The reversibility of the effects of MMPs on PHE-induced contraction suggests that the actions are not solely due to irreversible degradation of ECM. MMP-2 induced relaxation may not involve increased NO or PGI2 production, as it is not blocked by the NOS inhibitor L-NAME or the COX inhibitor indomethacin. Other studies on rats have shown that NO donors enhanced MMP-2 and -9 activities and NOS inhibitor reduced their activities in the maternal side of the placenta, demonstrating that NO may modulate the activation of MMPs (Pustovrh et al., 2007). MMP-2 induced venous relaxation is abolished by blockers of large conductance Ca2+-activated K+ channels such as iberiotoxin, suggesting involvement of VSM hyperpolarization pathway (Raffetto et al.2007).
MMPs do not inhibit PHE-induced VSM contraction in Ca2+-free solution implying that they do not inhibit the Ca2+ release mechanism. In contrast, MMPs inhibit PHE-induced Ca2+ influx (Chew et al., 2004). MMP-induced degradation of collagen produces Arg-Gly-Asp (RGD)-containing peptides, which bind to αvβ3 integrin receptors and inhibit Ca2+ entry into VSM (Waitkus-Edwards et al., 2002). Also, MMPs stimulate protease-activated receptors (PARs) and activate signaling pathways that could block VSM Ca2+ channels (Macfarlane et al.2001). This is supported by reports that proteases such as thrombin activate PARs and promote endothelium-dependent VSM relaxation by inhibiting Ca2+ influx (Hamilton et al.1998).
Clinical Applications and Perspectives
Ca2+ plays a major role in the regulation of vascular function particularly during pregnancy. Dietary, plasma and vascular cell Ca2+ are influenced by age, ethnic background and geographical distribution. An imbalance in the Ca2+ control mechanisms could significantly affect the outcome of pregnancy.
It appears that changes in [Ca2+]i in erythrocytes, platelets and immune cells as well as ECs and VSM cells play a role in PE. The differences in the results among various studies could be related to differences in the size of the study sub-groups. Also, whether the changes in vascular cell [Ca2+]i are a cause or consequence of PE remain to be clarified.
Although the results of different studies are not consistent, there is tangible evidence for beneficial effect of Ca2+-Suppl during pregnancy. Ca2+ may have the strongest beneficial effects in lowering the incidence of PE in women with inadequate Ca2+ intake. If Ca2+ intake is adequate, Ca2+-Suppl may not be needed.
Careful monitoring of vascular and cellular Ca2+ may also be important in the management of clinical cases of PE. Although the exact cause of the convulsion and excitation during eclampsia is not known, a role of Ca2+ is suspected. Magnesium sulfate is considered the first-line therapy to protect against seizures associated with PE-eclampsia, mainly because it prevents vascular spasm in the brain due to its Ca2+ antagonist properties. Other medication that could be considered in PE is Ca2+ antagonists, which could affect vasoconstriction, cytokine release, and MMPs. Ca2+ channel blockers have been tested in PE with positive effect (Walters & Redman, 1984, Papatsonis et al. 2001; Brown et al.2002, Elatrous et al. 2002, Fletcher et al., 1999, Hanff et al.2005). Intravenous diazoxide is one of the earliest drugs to be used in PE, but may cause sudden maternal hypotension, hyperglycemia and uterine atony as well as fetal distress and hypoglycaemia. The most common HTN therapy is nifedipine (given orally or sublingually) and hydralazine. Intravenous labtelalol and glyceryl trinitrate have also been considered. ACE inhibitors are contraindicated in pregnancy because of their harmful effects on the fetus. Further investigation of the regulation of vascular and cellular Ca2+ during pregnancy should help further delineate the causes of dysregulation of Ca2+ handling mechanisms during PE, and thereby identify cellular and molecular target for prevention and treatment of PE.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL-HL-65998, and HL-70659).
List of abbreviations
- 1,25(OH)2D3
1,25 dihydroxycholecalciferol, AngII, angiotensin II
- Ca2+
calcium
- [Ca2+]i
intracellular free Ca2+ concentration
- Ca2+-Suppl
Ca2+ supplementation
- EC
endothelial cell
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ET-1
endothelin-1
- HTN
hypertension
- HTN-Preg
HTN in pregnancy
- MMP
matrix metalloproteinase
- MAPK
mitogen-activated protein kinase
- MLC
myosin light chain
- NO
nitric oxide
- Norm-Preg
normal pregnant
- O2−•
superoxide
- PE
preeclampsia
- PGI2
prostacyclin
- PHE
phenylephrine
- PDGF
platelet-derived growth factor
- PAF
platelet-activating factor
- PGF
placenta growth factor
- PKC
protein kinase C
- PMN
polymorphonuclear neutrophils
- RCCT
randomized controlled clinical trial
- ROS
reactive oxygen species
- STBM
syncytiotrophoblast microvillous membranes
- TXA2
thromboxane A2
- VEGF
vascular endothelial growth factor
- VGCC
voltage-gated Ca2+ channel
- VSM
vascular smooth muscle
REFERENCES
- Abram SR, Alexander BT, Bennett WA, Granger JP. Role of neuronal nitric oxide synthase in mediating renal hemodynamic changes during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1390–3. doi: 10.1152/ajpregu.2001.281.5.R1390. [DOI] [PubMed] [Google Scholar]
- Adam LP, Gapinski CJ, Hathaway DR. Phosphorylation sequences in h-caldesmon from phorbol ester-stimulated canine aortas. FEBS Lett. 1992;302(3):223–6. doi: 10.1016/0014-5793(92)80446-n. [DOI] [PubMed] [Google Scholar]
- Aiko A, Ito M, Okamura H, Araki H, Nishi K. Effect of a low calcium intake on the vascular sensitivity to angiotensin II in normotensive pregnant rats. Artery. 1992;19(4):199–210. [PubMed] [Google Scholar]
- Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43:832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension. 1999;33(1):435–9. doi: 10.1161/01.hyp.33.1.435. [DOI] [PubMed] [Google Scholar]
- Allali F, El Aichaoui S, Khazani H, Benyahia B, Saoud B, El Kabbaj S, Bahiri R, Abouqal R, Hajjaj-Hassouni N. High Prevalence of Hypovitaminosis D in Morocco: Relationship to Lifestyle, Physical Performance, Bone Markers, and Bone Mineral Density. Semin Arthritis Rheum. 2008 Mar 11; doi: 10.1016/j.semarthrit.2008.01.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils are stimulated by syncytiotrophoblast icrovillous membranes to generate superoxide radicals in women with preeclampsia. Am J Obstet Gynecol. 2004;190(1):252–8. doi: 10.1016/j.ajog.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Anumba DOC, Robson SC, Boys RJ, Ford GA. Nitric oxide activity in the peripheral vasculature during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol. 1999;277(2):H848–H854. doi: 10.1152/ajpheart.1999.277.2.H848. [DOI] [PubMed] [Google Scholar]
- August P, Marcaccio B, Gertner JM, Druzin ML, Resnick LM, Laragh JH. Abnormal 1,25-dihydroxyvitamin D metabolism in preeclampsia. Am J Obstet Gynecol. 1992;166(4):1295–9. doi: 10.1016/s0002-9378(11)90625-5. [DOI] [PubMed] [Google Scholar]
- Baker PN, Davidge ST, Barankiewicz J, Roberts JM. Plasma of preeclamptic women stimulates and then inhibits endothelial prostacyclin. Hypertension. 1996;27(1):56–61. doi: 10.1161/01.hyp.27.1.56. [DOI] [PubMed] [Google Scholar]
- Baker PN, Davidge ST, Roberts JM. Plasma from women with pre-eclampsia increases endothelial cell nitric oxide production. Hypertension. 1995;26:244–248. doi: 10.1161/01.hyp.26.2.244. [DOI] [PubMed] [Google Scholar]
- Bar J, Ben-Haroush A, Lahav J, Sullivan M. Interaction Between Platelets and Cytokines - A Possible Role in the Pathogenesis of Preeclampsia. Vascular Disease Prevention. 2004;1(2):101–107. [Google Scholar]
- Barr SM, Lees KR, Butters L, O'Donnell A, Rubin PC. Platelet intracellular free calcium concentration in normotensive and hypertensive pregnancies in the human. Clin Sci (Lond) 1989;76(1):67–71. doi: 10.1042/cs0760067. [DOI] [PubMed] [Google Scholar]
- Baylis C. The determinants of renal hemodynamics in pregnancy. Am J Kidney Dis. 1987;9:260–4. doi: 10.1016/s0272-6386(87)80119-1. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82(5):1582–8. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- Boccardo P, Soregaroli M, Aiello S, Noris M, Donadelli R, Lojacono A, Benigni A. Systemic and fetal-maternal nitric oxide synthesis in normal pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1996;103(9):879–86. doi: 10.1111/j.1471-0528.1996.tb09906.x. [DOI] [PubMed] [Google Scholar]
- Bosio PM, Wheeler T, Anthony F, Conroy R, O'herlihy C, McKenna P. Maternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistance. Am J Obstet Gynecol. 2001;184(2):146–52. doi: 10.1067/mob.2001.108342. [DOI] [PubMed] [Google Scholar]
- Bouchlariotou S, Liakopoulos V, Dovas S, Giannopoulou M, Kiropoulos T, Zarogiannis S, Gatselos G, Zachopoulos T, Kyriakou DS, Kallitsaris A, Messinis I. Stefanidis I., Nocturnal hypertension is associated with an exacerbation of the endothelial damage in preeclampsia. Am J Nephrol. 2008;28(3):424–30. doi: 10.1159/000112807. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Manganella G, Thil-Trubert CL, Sachs C, Cournot G. Bioactive parathyroid hormone in pregnant rats and fetuses. Am J Physiol. 1990;258(4 Pt 1):E549–54. doi: 10.1152/ajpendo.1990.258.4.E549. [DOI] [PubMed] [Google Scholar]
- Brennecke SP, Gude NM, Di Iulio JL, King RG. Reduction of placental nitric oxide synthase activity in pre-eclampsia. Clin Sci (Lond) 1997;93(1):51–5. doi: 10.1042/cs0930051. [DOI] [PubMed] [Google Scholar]
- Bronner YL, Hawkins AS, Holt ML, Hossain MB, Rowel RH, Sydnor KL, Divers SP. Models for nutrition education to increase consumption of calcium and dairy products among African Americans. J Nutr. 2006;136(4):1103–6. doi: 10.1093/jn/136.4.1103. [DOI] [PubMed] [Google Scholar]
- Brown MA, Buddle ML, Farrell T, Davis GK. Efficacy and safety of nifedipine tablets for the acute treatment of severe hypertension in pregnancy. Am J Obstet Gynecol. 2002;187(4):1046–50. doi: 10.1067/mob.2002.126294. [DOI] [PubMed] [Google Scholar]
- Bucher HC, Guyatt GH, Cook RJ, Hatala R, Cook DJ, Lang JD, Hunt D. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275(14):1113–7. doi: 10.1001/jama.1996.03530380055031. [DOI] [PubMed] [Google Scholar]
- Burnand B, Sloutskis D, Gianoli F, Cornuz J, Rickenbach M, Paccaud F, Burckhardt P. Serum 25-hydroxyvitamin D: distribution and determinants in the Swiss population. Am J Clin Nutr. 1992;56(3):537–42. doi: 10.1093/ajcn/56.3.537. [DOI] [PubMed] [Google Scholar]
- Carrera F, Casart YC, Proverbio T, Proverbio F, Marín R. Preeclampsia and calcium-ATPase activity of plasma membranes from human myometrium and placental trophoblast. Hypertens Pregnancy. 2003;22(3):295–304. doi: 10.1081/PRG-120024033. [DOI] [PubMed] [Google Scholar]
- Ceyhan T, Beyan C, Başer I, Kaptan K, Güngör S, Ifran A. The effect of pre-eclampsia on complete blood count, platelet count and mean platelet volume. Ann Hematol. 2006;85(5):320–2. doi: 10.1007/s00277-006-0091-7. [DOI] [PubMed] [Google Scholar]
- Chen BC, Wu WT, Ho FM, Lin WW. Inhibition of Interleukin-1-induced NF-κB Activation by Calcium/Calmodulin-dependent Protein Kinase Kinase Occurs through Akt Activation Associated with Interleukin-1 Receptor-associated Kinase Phosphorylation and Uncoupling of MyD88. J Biol Chem. 2002;277(27):24169–24179. doi: 10.1074/jbc.M106014200. [DOI] [PubMed] [Google Scholar]
- Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40(5):1001–10. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37(3):240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Davis AK. Nitric oxide synthase activity in placentae from women with pre-eclampsia. Placenta. 1995;16(8):691–9. doi: 10.1016/0143-4004(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Crews JK, Novak J, Granger JP, Khalil RA. Stimulated mechanisms of Ca2+ entry into vascular smooth muscle during NO synthesis inhibition in pregnant rats. Am J Physiol. 1999;276(2):R530–8. doi: 10.1152/ajpregu.1999.276.2.R530. [DOI] [PubMed] [Google Scholar]
- Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61(3):514–23. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Hiller JE, Pridmore B, Bryce R, Duggan P, Hague WM, Robinson JS. Calcium supplementation in nulliparous women for the prevention of pregnancy-induced hypertension, preeclampsia and preterm birth: an Australian randomized trial. FRACOG and the ACT Study Group. Aust N Z J Obstet Gynaecol. 1999;39(1):12–8. doi: 10.1111/j.1479-828x.1999.tb03434.x. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Graceffa P, Wang CA, Wrangle J, Adam LP. Mammal-specific, ERK-dependent, caldesmon phosphorylation in smooth muscle. Quantitation using novel anti-phosphopeptide antibodies. J Biol Chem. 1999;274(42):30115–21. doi: 10.1074/jbc.274.42.30115. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Baker PN, Roberts JM. NOS expression is increased in endothelial cells exposed to plasma from women with preeclampsia. Am J Physiol. 1995;269:H1106–H1112. doi: 10.1152/ajpheart.1995.269.3.H1106. [DOI] [PubMed] [Google Scholar]
- Davidge ST, McLaughlin MK. Endogenous modulation of the blunted adrenergic response in resistance-sized mesenteric arteries from the pregnant rat. Am J Obstet Gynecol. 1992;167(6):1691–8. doi: 10.1016/0002-9378(92)91763-z. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Signorella AP, Hubel CA, Lykins DL, Roberts JM. Distinct factors in plasma of preeclamptic women increase endothelial nitric oxide or prostacyclin. Hypertension. 1996;28(5):758–64. doi: 10.1161/01.hyp.28.5.758. [DOI] [PubMed] [Google Scholar]
- Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R390–9. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- Davis OK, Hawkins DS, Rubin LP, Posillico JT, Brown EM, Schiff I. Serum parathyroid hormone (PTH) in pregnant women determined by an immunoradiometric assay for intact PTH. J Clin Endocrinol Metab. 1988;67(4):850–2. doi: 10.1210/jcem-67-4-850. [DOI] [PubMed] [Google Scholar]
- Davison JM, Homuth V, Jeyabalan A, Conrad KP, Karumanchi SA, Quaggin S, Dechend R, Luft FC. New aspects in the pathophysiology of preeclampsia. J Am Soc Nephrol. 2004;15(9):2440–8. doi: 10.1097/01.ASN.0000135975.90889.60. [DOI] [PubMed] [Google Scholar]
- Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101(20):2382–7. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- Deng A, Engels K, Baylis C. Impact of nitric oxide deficiency on blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int. 1996;50(4):1132–8. doi: 10.1038/ki.1996.420. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Peeters LL. Renal hemodynamics and volume homeostasis in pregnancy. Obstet Gynecol Surv. 1994;49(12):830–9. doi: 10.1097/00006254-199412000-00007. [DOI] [PubMed] [Google Scholar]
- Ebeigbe AB, Ezimokhai M, Aloamaka CP. Responses of arterial smooth muscle from normotensive and pre-eclamptic subjects to the calcium channel agonist, Bay K 8644. Res Exp Med (Berl) 1987;187(6):461–8. doi: 10.1007/BF01852184. [DOI] [PubMed] [Google Scholar]
- Ebose EJ, Campbell PI, Okorodudu AO. Electrolytes and pH changes in pre-eclamptic rats. Clin Chim Acta. 2007;384(1-2):135–40. doi: 10.1016/j.cca.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Elatrous S, Nouira S, Ouanes Besbes L, Marghli S, Boussarssar M, Sakkouhi M, Abroug F. Short-term treatment of severe hypertension of pregnancy: prospective comparison of nicardipine and labetalol. Intensive Care Med. 2002;28(9):1281–6. doi: 10.1007/s00134-002-1406-3. [DOI] [PubMed] [Google Scholar]
- Ezimokhai M, Osman N. Calcium supplementation is associated with endothelium dependent attenuation of vascular smooth muscle reactivity in normotensive pregnant and nonpregnant rats. Am J Hypertens. 1998;11(1 Pt 1):88–96. doi: 10.1016/s0895-7061(97)00367-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Stewart KG, Zhang Y, Koivunen E, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ Res. 2000;87(8):670–6. doi: 10.1161/01.res.87.8.670. [DOI] [PubMed] [Google Scholar]
- Fischer D, Schroer A, Lüdders D, Cordes T, Bücker B, Reichrath J, Friedrich M. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin Exp Obstet Gynecol. 2007;34(2):80–4. [PubMed] [Google Scholar]
- Fletcher H, Roberts G, Mullings A, Forrester T. An open trial comparing isradipine with hydralazine and methyl dopa in the treatment of patients with severe pre-eclampsia. J Obstet Gynaecol. 1999;19(3):235–8. doi: 10.1080/01443619964977. [DOI] [PubMed] [Google Scholar]
- Friel AM, Sexton DJ, O'reilly MW, Smith TJ, Morrison JJ. Rho A/Rho kinase: human umbilical artery mRNA expression in normal and pre eclamptic pregnancies and functional role in isoprostane-induced vasoconstriction. Reproduction. 2006;132(1):169–76. doi: 10.1530/rep.1.01088. [DOI] [PubMed] [Google Scholar]
- Fukuoka H, Haruna M. Calcium and bone metabolism during pregnancy and lactation. Clin Calcium. 2003;13(11):1425–31. [PubMed] [Google Scholar]
- Galewska Z, Bańkowski E, Romanowicz L, Jaworski S. Pre-eclampsia (EPH-gestosis)-induced decrease of MMP-s content in the umbilical cord artery. Clin Chim Acta. 2003;335(1-2):109–15. doi: 10.1016/s0009-8981(03)00296-1. [DOI] [PubMed] [Google Scholar]
- Gallery ED, Campbell S, Arkell J, Nguyen M, Jackson CJ. Preeclamptic decidual microvascular endothelial cells express lower levels of matrix metalloproteinase-1 than normals. Microvasc Res. 1999;57(3):340–6. doi: 10.1006/mvre.1998.2142. [DOI] [PubMed] [Google Scholar]
- Gallery ED, Rowe J, Campbell S, Hawkins T. Effect of serum on secretion of prostacyclin and endothelin-1 by decidual endothelial cells from normal and preeclamptic pregnancies. Am J Obstet Gynecol. 1995;173:918–923. doi: 10.1016/0002-9378(95)90366-6. [DOI] [PubMed] [Google Scholar]
- Giambelluca MS, Gende OA. Hydrogen peroxide activates calcium influx in human neutrophils. Mol Cell Biochem. 2008;309(1-2):151–6. doi: 10.1007/s11010-007-9653-9. [DOI] [PubMed] [Google Scholar]
- Giardina JB, Green GM, Cockrell KL, Granger JP, Khalil RA. TNF-alpha enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R130–43. doi: 10.1152/ajpregu.00704.2001. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- Gillette ME, Insogna KL, Lewis AM, Baran DT. Influence of pregnancy on immunoreactive parathyroid hormone levels. Calcif Tissue Int. 1982;34(1):9–12. doi: 10.1007/BF02411200. [DOI] [PubMed] [Google Scholar]
- Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol. 2006;290(5):H2124–35. doi: 10.1152/ajpheart.00813.2005. [DOI] [PubMed] [Google Scholar]
- Gonen E, Sahin I, Ozbek M, Kovalak E, Yologlu S, Ates Y. Effects of pregnancy and lactation on bone mineral density, and their relation to the serum calcium, phosphorus, calcitonin and parathyroid hormone levels in rats. J Endocrinol Invest. 2005;28(4):322–6. doi: 10.1007/BF03347197. [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38(3 Pt 2):718–22. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9(3):147–60. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- Green J, Assady S, Nakhoul F, Bick T, Jakobi P, Abassi Z. Differential effects of sera from normotensive and hypertensive pregnant women on Ca2+ metabolism in normal vasular smooth muscle cells. J Am Soc Nephrol. 2000;11(7):1188–98. doi: 10.1681/ASN.V1171188. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gebhardt S, Hillermann R, Holzgreve W, Hahn S. Analysis of plasma elastase levels in early and late onset preeclampsia. Arch Gynecol Obstet. 2006;273(4):239–42. doi: 10.1007/s00404-005-0093-z. [DOI] [PubMed] [Google Scholar]
- Halhali A, Díaz L, Avila E, Ariza AC, Garabédian M, Larrea F. Decreased fractional urinary calcium excretion and serum 1,25-dihydroxyvitamin D and IGF-I levels in preeclampsia. J Steroid Biochem Mol Biol. 2007;103(3-5):803–6. doi: 10.1016/j.jsbmb.2006.12.055. [DOI] [PubMed] [Google Scholar]
- Haller H, Hempel A, Homuth V, Mandelkow A, Maasch C, Drab M, Lindschau C, Vetter K, Dudenhausen J, Luft FC. Endothelial cell permeability and protein kinase C in preeclampsia. Lancet. 1998;351:945–949. doi: 10.1016/S0140-6736(05)60605-8. [DOI] [PubMed] [Google Scholar]
- Haller H, Oeney T, Hauck U, Distler A, Philipp T. Increased intracellular free calcium and sensitivity to angiotensin II in platelets of preeclamptic women. Am J Hypertens. 1989;2(4):238–43. doi: 10.1093/ajh/2.4.238. [DOI] [PubMed] [Google Scholar]
- Haller H, Ziegler EM, Homuth V, Drab M, Eichhorn J, Nagy Z, Busjahn A, Vetter K, Luft FC. Endothelial adhesion molecules and leukocyte integrins in preeclamptic patients. Hypertension. 1997;29(1):291–6. doi: 10.1161/01.hyp.29.1.291. [DOI] [PubMed] [Google Scholar]
- Hamilton JR, Nguyen PB, Cocks TM. Atypical protease-activated receptor mediates endothelium-dependent relaxation of human coronary arteries. Circ Res. 1998;82(12):1306–11. doi: 10.1161/01.res.82.12.1306. [DOI] [PubMed] [Google Scholar]
- Hanff LM, Vulto AG, Bartels PA, Roofthooft DW, Bijvank BN, Steegers EA, Visser W. Intravenous use of the calcium-channel blocker nicardipine as second-line treatment in severe, early-onset pre-eclamptic patients. J Hypertens. 2005;23(12):2319–26. doi: 10.1097/01.hjh.0000188729.73807.16. [DOI] [PubMed] [Google Scholar]
- Harville EW, Schramm M, Watt-Morse M, Chantala K, Anderson JJ, Hertz-Picciotto I. Calcium intake during pregnancy among white and African-American pregnant women in the United States. J Am Coll Nutr. 2004;23(1):43–50. doi: 10.1080/07315724.2004.10719341. [DOI] [PubMed] [Google Scholar]
- Hawkins T, Jones MP, Gallery ED. Secretion of prostanoids by platelets and monocytes in normal and hypertensive pregnancies. Am J Obstet Gynecol. 1993;168(2):661–7. doi: 10.1016/0002-9378(93)90514-j. [DOI] [PubMed] [Google Scholar]
- Hedges JC, Oxhorn BC, Carty M, Adam LP, Yamboliev IA, Gerthoffer WT. Phosphorylation of caldesmon by ERK MAP kinases in smooth muscle. Am J Physiol Cell Physiol. 2000;278(4):C718–26. doi: 10.1152/ajpcell.2000.278.4.C718. [DOI] [PubMed] [Google Scholar]
- Herrera JA, Arévalo-Herrera M, Shahabuddin AK, Ersheng G, Herrera S, Garcia RG, López-Jaramillo P. Calcium and conjugated linoleic acid reduces pregnancy-induced hypertension and decreases intracellular calcium in lymphocytes. Am J Hypertens. 2006;19(4):381–7. doi: 10.1016/j.amjhyper.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG. 2007;114(8):933–43. doi: 10.1111/j.1471-0528.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- Hojo M, Suthanthiran M, Helseth G, August P. Lymphocyte intracellular free calcium concentration is increased in preeclampsia. Am J Obstet Gynecol. 1999;180(5):1209–14. doi: 10.1016/s0002-9378(99)70618-6. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76(4):967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Hu E. Recent patents on Rho signaling pathway as therapeutic target for cardiovascular diseases. Recent Patents Cardiovasc Drug Discov. 2006;1(3):249–63. doi: 10.2174/157489006778777034. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu MQ, Li H, Xu C, Yi SH, Chen GH. Occurrence of cGMP/nitric oxide-sensitive store-operated calcium entry in fibroblasts and its effect on matrix metalloproteinase secretion. World J Gastroenterol. 2006;12(34):5483–9. doi: 10.3748/wjg.v12.i34.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman MA, Timmer A, Zeinstra M, Serlier EK, Hanemaaijer R, Goor H, Erwich JJ. Matrix-metalloproteinase activity in first trimester placental bed biopsies in further complicated and uncomplicated pregnancies. Placenta. 2004;25(4):253–8. doi: 10.1016/j.placenta.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Janes SL, Kyle PM, Redman C, Goodall AH. Flow cytometric detection of activated platelets in pregnant women prior to the development of pre-eclampsia. Thromb Haemost. 1995;74(4):1059–63. [PubMed] [Google Scholar]
- Javed MU, Naru T, Michelangeli F. An endogenous inhibitor of Ca++-ATPase from human placenta. J Enzyme Inhib. 2000;15(2):163–70. doi: 10.1080/14756360009030348. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93(12):1249–57. doi: 10.1161/01.RES.0000104086.43830.6C. [DOI] [PubMed] [Google Scholar]
- Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287(4):H1495–500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87(1):136–41. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Alexander BT, Granger JP, Khalil RA. Ca2+-insensitive vascular protein kinase C during pregnancy and NOS inhibition. Hypertension. 1999;34(4 Pt 2):924–30. doi: 10.1161/01.hyp.34.4.924. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R295–303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney Int. 2007;71(10):959–61. doi: 10.1038/sj.ki.5002281. [DOI] [PubMed] [Google Scholar]
- Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important Role of Rho-kinase in the Pathogenesis of Cardiovascular Inflammation and Remodeling Induced by Long-Term Blockade of Nitric Oxide Synthesis in Rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- Kaur G, Mishra S, Sehgal A, Prasad R. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol Cell Biochem. 2008;333:37–44. doi: 10.1007/s11010-008-9739-z. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;15(90(22)):10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31(5):1065–9. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Menice CB, Wang CL, Morgan KG. Phosphotyrosine-dependent targeting of mitogen-activated protein kinase in differentiated contractile vascular cells. Circ Res. 1995;76(6):1101–8. doi: 10.1161/01.res.76.6.1101. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Broughton Pipkin F, Cockbill S, Heptinstall S, Symonds EM. A cross-sectional study of basal platelet intracellular free calcium concentration in normotensive and hypertensive primigravid pregnancies. Clin Sci (Lond) 1990;78(1):75–80. doi: 10.1042/cs0780075. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Broughton Pipkin F, Symonds EM. Platelet cytosolic calcium in human pregnancy complicated by essential hypertension. Am J Obstet Gynecol. 1993;169(1):141–3. doi: 10.1016/0002-9378(93)90149-d. [DOI] [PubMed] [Google Scholar]
- Kisters K, Barenbrock M, Louwen F, Hausberg M, Rahn KH, Kosch M. Membrane, intracellular, and plasma magnesium and calcium concentrations in preeclampsia. Am J Hypertens. 2000;13:765–9. doi: 10.1016/s0895-7061(00)00240-5. [DOI] [PubMed] [Google Scholar]
- Kisters K, Barenbrocka M, Louwenb F, Hausberga M, Rahna KH, Koscha M. Membrane, intracellular, and plasma magnesium and calcium concentrations in preeclampsia. Am J Hypertens. 2000;13(7):765–9. doi: 10.1016/s0895-7061(00)00240-5. [DOI] [PubMed] [Google Scholar]
- Knight KB, Keith RE. Calcium supplementation on normotensive and hypertensive pregnant women. Am J Clin Nutr. 1992;55(4):891–5. doi: 10.1093/ajcn/55.4.891. [DOI] [PubMed] [Google Scholar]
- Ko HM, Kang JH, Choi JH, Park SJ, Bai S, Im SY. Platelet-activating factor induces matrix metalloproteinase-9 expression through Ca2+- or PI3K-dependent signaling pathway in a human vascular endothelial cell line. FEBS Lett. 2005;579(28):6451–8. doi: 10.1016/j.febslet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88(5):2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- Konijnenberg A, Stokkers EW, van der Post JA, Schaap MC, Boer K, Bleker OP, Sturk A. Extensive platelet activation in preeclampsia compared with normal pregnancy: enhanced expression of cell adhesion molecules. Am J Obstet Gynecol. 1997;176(2):461–9. doi: 10.1016/s0002-9378(97)70516-7. [DOI] [PubMed] [Google Scholar]
- Kosch M, Hausberg M, Louwen F, Barenbrock M, Rahn KH, Kisters K. Alterations of plasma calcium and intracellular and membrane calcium in erythrocytes of patients with pre-eclampsia. J Hum Hypertens. 2000;14(5):333–6. doi: 10.1038/sj.jhh.1001006. [DOI] [PubMed] [Google Scholar]
- Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Tamaki T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol Sin. 2004;25(8):977–85. [PubMed] [Google Scholar]
- Kyle PM, Jackson MC, Buckley DC, de Swiet M, Redman CW. Platelet intracellular free calcium response to arginine vasopressin is similar in preeclampsia and normal pregnancy. Am J Obstet Gynecol. 1995;172(2 Pt 1):654–60. doi: 10.1016/0002-9378(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Lamarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9(6):480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- Lantoine F, Iouzalen L, Devynck MA, Millanvoye-Van Brussel E, David-Dufilho M. Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochem J. 1998;330(Pt 2):695–9. doi: 10.1042/bj3300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Quinn PA, Jennings SC, Ng LL. Neutrophil activation and production of reactive oxygen species in pre-eclampsia. Hypertension. 2003;21(2):395–402. doi: 10.1097/00004872-200302000-00032. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, DerSimonian R, Esterlitz JR, Raymond EG, Bild DE, Clemens JD, Cutler JA. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol. 2000;183(6):1554–7. doi: 10.1067/mob.2000.108022. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF, Schatz F. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78(6):1064–72. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G, Guérin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98(3):322–34. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- López-Jaramillo P, Delgado F, Jácome P, Terán E, Ruano C, Rivera J. Calcium supplementation and the risk of preeclampsia in Ecuadorian pregnant teenagers. Obstet Gynecol. 1997;90(2):162–7. doi: 10.1016/S0029-7844(97)00254-8. [DOI] [PubMed] [Google Scholar]
- Luppi P, Tse H, Lain KY, Markovic N, Piganelli JD, DeLoia JA. Preeclampsia activates circulating immune cells with engagement of the NF-kappaB pathway. Am J Reprod Immunol. 2006;56(2):135–44. doi: 10.1111/j.1600-0897.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Lurie S, Frenkel E, Tuvbin Y. Comparison of the differential distribution of leukocytes in preeclampsia versus uncomplicated pregnancy. Gynecol Obstet Invest. 1998;45(4):229–31. doi: 10.1159/000009973. 1. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–82. [PubMed] [Google Scholar]
- MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260(3):E464–70. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- Mahameed S, Goldman S, Gabarin D, Weiss A, Shalev E. The effect of serum from women with preeclampsia on JAR (trophoblast-like) cell line. J Soc Gynecol Investig. 2005;12(6):e45–50. doi: 10.1016/j.jsgi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10(11):370–5. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Merchant SJ, Narumiya H, Zhang Y, Guilbert LJ, Davidge ST. The effects of preeclampsia and oxygen environment on endothelial release of matrix metalloproteinase-2. Hypertens Pregnancy. 2004;23(1):47–60. doi: 10.1081/PRG-120028281. [DOI] [PubMed] [Google Scholar]
- Mizutani S, Tomoda Y. Effects of placental proteases on maternal and fetal blood pressure in normal pregnancy and preeclampsia. Am J Hypertens. 1996;9(6):591–7. doi: 10.1016/0895-7061(96)00016-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res. 2006;31(98(6)):743–56. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. [Ca2+]i signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R87–99. doi: 10.1152/ajpregu.2001.280.1.R87. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol. 2003;284(1):H393–403. doi: 10.1152/ajpheart.00247.2002. [DOI] [PubMed] [Google Scholar]
- Nardulli G, Proverbio F, Limongi FG, Marín R, Proverbio T. Preeclampsia and calcium adenosine triphosphatase activity of red blood cell ghosts. Am J Obstet Gynecol. 1994;171(5):1361–5. doi: 10.1016/0002-9378(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Narumiya H, Zhang Y, Fernandez-Patron C, Guilbert LJ, Davidge ST. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20:185–94. doi: 10.1081/PRG-100106968. [DOI] [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res. 2000;87(5):406–11. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81(4):1415–59. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, Macconi D, Maucci R, Porrati F, Benigni A, Picciolo C, Remuzzi G. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004;43(3):614–22. doi: 10.1161/01.HYP.0000116220.39793.c9. [DOI] [PubMed] [Google Scholar]
- Oken E, Ning Y, Rifas-Shiman SL, Rich-Edwards JW, Olsen SF, Gillman MW. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann Epidemiol. 2007;17(9):663–8. doi: 10.1016/j.annepidem.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension. 2004;43(2):434–44. doi: 10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1013–23. doi: 10.1152/ajpregu.00729.2003. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Benaim G, Cervino V, Proverbio T, Proverbio F, Marín R. The plasma membrane Ca2+-ATPase protein from red blood cells is not modified in preeclampsia. Biochim Biophys Acta. 2006;1762(3):381–5. doi: 10.1016/j.bbadis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Papatsonis DN, Lok CA, Bos JM, Geijn HP, Dekker GA. Calcium channel blockers in the management of preterm labor and hypertension in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;97(2):122–40. doi: 10.1016/s0301-2115(00)00548-0. [DOI] [PubMed] [Google Scholar]
- Papazoglou D, Galazios G, Koukourakis MI, Panagopoulos I, Kontomanolis EN, Papatheodorou K, Maltezos E. Vascular endothelial growth factor gene polymorphisms and pre-eclampsia. Mol Hum Reprod. 2004;10(5):321–4. doi: 10.1093/molehr/gah048. [DOI] [PubMed] [Google Scholar]
- Pedersen EB, Johannesen P, Kristensen S, Rasmussen AB, Emmertsen K, Møller J, Lauritsen JG, Wohlert M. Calcium, parathyroid hormone and calcitonin in normal pregnancy and preeclampsia. Gynecol Obstet Invest. 1984;18(3):156–64. doi: 10.1159/000299073. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- Pérez-López FR. Vitamin D: the secosteroid hormone and human reproduction. Gynecol Endocrinol. 2007;23(1):13–24. doi: 10.1080/09513590601045629. [DOI] [PubMed] [Google Scholar]
- Prada JA, Tsang RC, Clark KE. Hypocalcemia and pregnancy-induced hypertension produced by low-calcium diet. Hypertension. 1994;23(6):695–702. doi: 10.1161/01.hyp.23.6.695. [DOI] [PubMed] [Google Scholar]
- Pustovrh MC, Jawerbaum A, White V, Capobianco E, Higa R, Martínez N, López-Costa JJ, González E. The role of nitric oxide on matrix metalloproteinase 2 (MMP2) and MMP9 in placenta and fetus from diabetic rats. Reproduction. 2007;134(4):605–13. doi: 10.1530/REP-06-0267. [DOI] [PubMed] [Google Scholar]
- Qiao C, Wang CH, Shang T, Lin QD. Clinical significance of KiSS-1 and matrix metalloproteinase-9 expression in trophoblasts of women with preeclampsia and their relation to perinatal outcome of neonates. Zhonghua Fu Chan Ke Za Zhi. 2005;40(9):585–90. [PubMed] [Google Scholar]
- Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–80. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón de Berrazueta J. The role of calcium in the regulation of normal vascular tone and in arterial hypertension. Rev Esp Cardiol. 1999;52(3):25–33. [PubMed] [Google Scholar]
- Ramos JG, Brietzke E, Martins-Costa SH, Vettorazzi-Stuczynski J, Barros E, Carvalho C. Reported calcium intake is reduced in women with preeclampsia. Hypertens Pregnancy. 2006;25(3):229–39. doi: 10.1080/10641950600913008. [DOI] [PubMed] [Google Scholar]
- Ray J, Vasishta K, Kaur S, Majumdar S, Sawhney H. Calcium metabolism in pre-eclampsia. Int J Gynaecol Obstet. 1999;66(3):245–50. doi: 10.1016/s0020-7292(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Richards SR, Nelson DM, Zuspan FP. Calcium levels in normal and hypertensive pregnant patients. Am J Obstet Gynecol. 1984;149(2):168–71. doi: 10.1016/0002-9378(84)90191-1. [DOI] [PubMed] [Google Scholar]
- Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67(4):693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- Rowe J, Campbell S, Gallery ED. Nitric oxide production by decidual endothelial cells is not reduced in preeclampsia. Hypertens Pregnancy. 2003;22(1):63–75. doi: 10.1081/PRG-120017005. [DOI] [PubMed] [Google Scholar]
- Saggese G, Baroncelli GI, Bertelloni S, Cipolloni C. Intact parathyroid hormone levels during pregnancy, in healthy term neonates and in hypocalcemic preterm infants. Acta Paediatr Scand. 1991;80(1):36–41. doi: 10.1111/j.1651-2227.1991.tb11726.x. [DOI] [PubMed] [Google Scholar]
- Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70(11):1537–47. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramos L, Jones DC, Cullen MT. Urinary calcium as an early marker for preeclampsia. Obstet Gynecol. 1991;77(5):685–8. [PubMed] [Google Scholar]
- Saraswathi V, Wu G, Toborek M, Hennig B. Linoleic acid-induced endothelial activation: role of calcium and peroxynitrite signaling. J Lipid Res. 2004;45(5):794–804. doi: 10.1194/jlr.M300497-JLR200. [DOI] [PubMed] [Google Scholar]
- Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511–1517. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. Calcium flux in neutrophils synchronizes beta2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng. 2008;36(4):632–46. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely EW, Wood RJ, Brown EM, Graves SW. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J Clin Endocrinol Metab. 1992;74(6):1436–40. doi: 10.1210/jcem.74.6.1592891. [DOI] [PubMed] [Google Scholar]
- Seki K, Makimura N, Mitsui C, Hirata J, Nagata I. Calcium-regulating hormones and osteocalcin levels during pregnancy: a longitudinal study. Am J Obstet Gynecol. 1991;164(5):1248–52. doi: 10.1016/0002-9378(91)90694-m. [DOI] [PubMed] [Google Scholar]
- Sheu JR, Hsiao G, Shen MY, Lin WY, Tzeng CR. The hyperaggregability of platelets from normal pregnancy is mediated through thromboxane A2 and cyclic AMP pathways. Clin Lab Haematol. 2002;24(2):121–9. doi: 10.1046/j.1365-2257.2002.t01-1-00430.x. [DOI] [PubMed] [Google Scholar]
- Shim JY, Jun JK, Jung BK, Kim SH, Won HS, Lee PR, Kim A. Vascular endothelial growth factor gene +936 C/T polymorphism is associated with preeclampsia in Korean women. Am J Obstet Gynecol. 2007;197(3):271. doi: 10.1016/j.ajog.2007.06.045. 1. [DOI] [PubMed] [Google Scholar]
- Shima E, Katsube M, Kato T, Kitagawa M, Hato F, Hino M, Takahashi T, Fujita H, Kitagawa S. Calcium channel blockers suppress cytokine-induced activation of human neutrophils. Am J Hypertens. 2008;21(1):78–84. doi: 10.1038/ajh.2007.13. [DOI] [PubMed] [Google Scholar]
- Silver HM, Seebeck M, Carlson R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurement of red blood cell and plasma volumes. Am J Obstet Gynecol. 1998;179(1):87–93. doi: 10.1016/s0002-9378(98)70255-8. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272(2):R441–63. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Smárason AK, Sargent IL, Starkey PM, Redman CW. The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br J Obstet Gynaecol. 1993;100(10):943–9. doi: 10.1111/j.1471-0528.1993.tb15114.x. [DOI] [PubMed] [Google Scholar]
- Szmidt-Adjidé V, Vendittelli F, David S, Brédent-Bangou J, Janky E. Calciuria and preeclampsia: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2006;125(2):193–8. doi: 10.1016/j.ejogrb.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Sowers JR, Zemel MB, Bronsteen RA, Zemel PC, Walsh MF, Standley PR, Sokol RJ. Erythrocyte cation metabolism in preeclampsia. Am J Obstet Gynecol. 1989;161(2):441–5. doi: 10.1016/0002-9378(89)90539-5. [DOI] [PubMed] [Google Scholar]
- Sowers JR, Zemel MB, Walsh MF, Standley PR, Zemel PC, Bronsteen RA, Kraniak J, Sokol RJ. Effects of normal pregnancy on cellular cation metabolism and peripheral vascular resistance. Am J Hypertens. 1990;3(1):16–22. doi: 10.1093/ajh/3.1.16. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Wallace RB, Lemke JH. The association of intakes of vitamin D and calcium with blood pressure among women. Am J Clin Nutr. 1985;42(1):135–42. doi: 10.1093/ajcn/42.1.135. [DOI] [PubMed] [Google Scholar]
- Steinckwich N, Frippiat JP, Stasia MJ, Erard M, Boxio R, Tankosic C, Doignon I, Nüsse O. Potent inhibition of store-operated Ca2+ influx and superoxide production in HL60 cells and polymorphonuclear neutrophils by the pyrazole derivative BTP2. J Leukoc Biol. 2007;81:1054–64. doi: 10.1189/jlb.0406248. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Wyatt AW, Poston L, Jacob R, Mann GE. Preeclampsia is associated with altered Ca2+ regulation and NO production in human fetal venous endothelial cells. FASEB J. 2002;16(7):721–3. doi: 10.1096/fj.01-0916fje. [DOI] [PubMed] [Google Scholar]
- Stennett AK, Khalil RA. Neurovascular mechanisms of hypertension in pregnancy. Curr Neurovasc Res. 2006;3(2):131–48. doi: 10.2174/156720206776875885. [DOI] [PubMed] [Google Scholar]
- Storey ML, Forshee RA, Anderson PA. Associations of adequate intake of calcium with diet, beverage consumption, and demographic characteristics among children and adolescents. J Am Coll Nutr. 2004;23(1):18–33. doi: 10.1080/07315724.2004.10719339. [DOI] [PubMed] [Google Scholar]
- Sukonpan K, Phupong V. Serum calcium and serum magnesium in normal and preeclamptic pregnancy. Arch Gynecol Obstet. 2005;273(1):12–6. doi: 10.1007/s00404-004-0672-4. [DOI] [PubMed] [Google Scholar]
- Tayebjee MH, Karalis I, Nadar SK, Beevers DG, MacFadyen RJ, Lip GY. Circulating matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases-1 and -2 levels in gestational hypertension. Am J Hypertens. 2004;17:162A–163A. doi: 10.1016/j.amjhyper.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Casal DC, Jones LA, Varma M, Martin JN, Jr, Roberts JM. Selective effects of preeclamptic sera on human endothelial cell procoagulant protein expression. Am J Obstet Gynecol. 1991;165:1705–1710. doi: 10.1016/0002-9378(91)90019-n. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Musci TJ, Rodgers GM, Roberts JM. Preeclamptic sera stimulate increased platelet-derived growth factor mRNA and protein expression by cultured human endothelial cells. Am J Reprod Immunol. 1991;25:105–108. doi: 10.1111/j.1600-0897.1991.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Teppa-Garrán A, Proverbio T, Marín R, Proverbio F. Lipid peroxidation and active calcium transport in inside-out vesicles of red blood cells from preeclamptic women. Int J Biochem Cell Biol. 2004;36(5):806–13. doi: 10.1016/j.biocel.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Teran E, Escudero C, Vivero S, Enriquez A, Calle A. Intraplatelet cyclic guanosine-3′,5′-monophosphate levels during pregnancy and preeclampsia. Hypertens Pregnancy. 2004;23(3):303–8. doi: 10.1081/PRG-200030860. [DOI] [PubMed] [Google Scholar]
- Thomas M, Weisman SM. Calcium supplementation during pregnancy and lactation: effects on the mother and the fetus. Am J Obstet Gynecol. 2006;194(4):937–45. doi: 10.1016/j.ajog.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110(12):1612–9. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- Tintinger GR, Theron AJ, Potjo M, Anderson R. Reactive oxidants regulate membrane depolarization and store-operated uptake of calcium by formyl peptide-activated human neutrophils. Free Radic Biol Med. 2007;42(12):1851–7. doi: 10.1016/j.freeradbiomed.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Torres PJ, Escolar G, Palacio M, Gratacós E, Alonso PL, Ordinas A. Platelet sensitivity to prostaglandin E1 inhibition is reduced in pre-eclampsia but not in nonproteinuric gestational hypertension. Br J Obstet Gynaecol. 1996;103(1):19–24. doi: 10.1111/j.1471-0528.1996.tb09510.x. [DOI] [PubMed] [Google Scholar]
- Trumbo PR, Ellwood KC. Supplemental calcium and risk reduction of hypertension, pregnancy-induced hypertension, and preeclampsia: an evidence-based review by the US Food and Drug Administration. Nutr Rev. 2007;65(2):78–87. doi: 10.1111/j.1753-4887.2007.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88(11):5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- van der Post JA, Konijnenberg A, Boer K, Schaap MC, van Boxtel CE, Sturk A, Boer GJ, Swaab DF. Preeclampsia is not associated with altered platelet vasopressin binding and cytosolic Ca++ concentration. Am J Obstet Gynecol. 1993;169(5):1169–78. doi: 10.1016/0002-9378(93)90276-o. [DOI] [PubMed] [Google Scholar]
- Van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- VanWijk MJ, Boer K, van der Meulen ET, Bleker OP, Spaan JA, VanBavel E. Resistance artery smooth muscle function in pregnancy and preeclampsia. Am J Obstet Gynecol. 2002;186(1):148–54. doi: 10.1067/mob.2002.119184. [DOI] [PubMed] [Google Scholar]
- Vaughan JE, Walsh SW. Neutrophils from pregnant women produce thromboxane and tumor necrosis factor-alpha in response to linoleic acid and oxidative stress. Am J Obstet Gynecol. 2005;193(3):830–5. doi: 10.1016/j.ajog.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Villar J, Abdel-Aleem H, Merialdi M, Mathai M, Ali MM, Zavaleta N, Purwar M, Hofmeyr J, Nguyen TN, Campódonico L, Landoulsi S, Carroli G, Lindheimer M, World Health Organization Calcium Supplementation for the Prevention of Preeclampsia Trial Group World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194(3):639–49. doi: 10.1016/j.ajog.2006.01.068. [DOI] [PubMed] [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102(1):20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Hurst G, Redman CW. Supernatants from co-cultured endothelial cells and syncytiotrophoblast microvillous membranes activate peripheral blood leukocytes in vitro. Hum Reprod. 1999;14(4):919–24. doi: 10.1093/humrep/14.4.919. [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Wilkins T, Redman CW. Maternal peripheral blood leukocytes in normal and pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1999;106(6):576–81. doi: 10.1111/j.1471-0528.1999.tb08327.x. [DOI] [PubMed] [Google Scholar]
- Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, Meininger GA. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90(4):473–80. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BN, Redman CW. Treatment of severe pregnancy-associated hypertension with the calcium antagonist nifedipine. Br J Obstet Gynaecol. 1984;91(4):330–6. doi: 10.1111/j.1471-0528.1984.tb05918.x. [DOI] [PubMed] [Google Scholar]
- Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91(11):5212–6. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead M, Lane G, Young O, Campbell S, Abeyasekera G, Hillyard CJ, MacIntyre I, Phang KG, Stevenson JC. Interrelations of calcium-regulating hormones during normal pregnancy. Br Med J (Clin Res Ed) 1981;283(6283):10–2. doi: 10.1136/bmj.283.6283.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczyński JR, Tchórzewski H, Głowacka E, Banasik M, Lewkowicz P, Szpakowski M, Zeman K, Wilczyński J. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators Inflamm. 2002;11(2):105–11. doi: 10.1080/09629350220131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Mahomed K, Farrand A, Woelk GB, Mudzamiri S, Madzime S, King IB, McDonald GB. Plasma tumor necrosis factor-alpha soluble receptor p55 (sTNFp55) concentrations in eclamptic, preeclamptic and normotensive pregnant Zimbabwean women. J Reprod Immunol. 1998;40(2):159–73. doi: 10.1016/s0165-0378(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Wimalasundera RC, Thom SA, Regan L, Hughes AD. Effects of vasoactive agents on intracellular calcium and force in myometrial and subcutaneous resistance arteries isolated from preeclamptic, pregnant, and nonpregnant woman. Am J Obstet Gynecol. 2005;192(2):625–32. doi: 10.1016/j.ajog.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169(5):1316–20. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]
- Vargas Zapata CL, Donangelo CM, Woodhouse LR, Abrams SA, Spencer EM, King JC. Calcium homeostasis during pregnancy and lactation in Brazilian women with low calcium intakes: a longitudinal study. Am J Clin Nutr. 2004;80(2):417–22. doi: 10.1093/ajcn/80.2.417. [DOI] [PubMed] [Google Scholar]
- Zemel MB, Zemel PC, Berry S, Norman G, Kowalczyk C, Sokol RJ, Standley PR, Walsh MF, Sowers JR. Altered platelet calcium metabolism as an early predictor of increased peripheral vascular resistance and preeclampsia in urban black women. N Engl J Med. 1990;323:434–8. doi: 10.1056/NEJM199008163230702. [DOI] [PubMed] [Google Scholar]