Fig. 8.
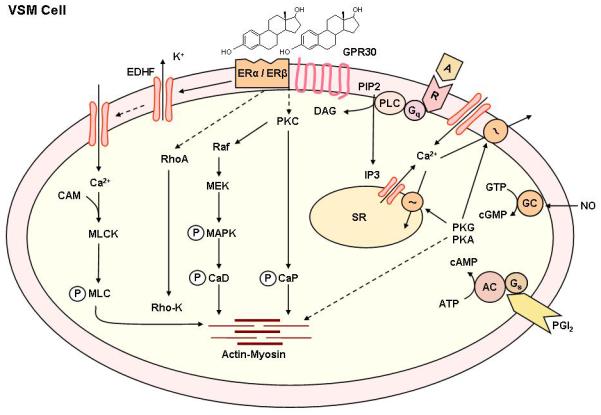
Non-genomic estrogen mediated mechanisms of VSM contraction. Agonists activate specific VSM receptors and stimulates PLC, and increases the production of IP3 and diacylglycerol (DAG). IP3 stimulates Ca2+ release from the sarcoplasmic reticulum (SR). Agonists also stimulate Ca2+ entry through Ca2+ channels. Ca2+ binds CAM, activates myosin light chain (MLC) kinase, causes MLC phosphorylation, and initiates VSM contraction. DAG causes activation of PKC, and PKC then phosphorylates calponin (CaP) and/or activate a protein kinase cascade involving Raf, MAPK kinase (MEK), and MAPK, leading to phosphorylation of caldesmon (CaD) and an increase in the myofilament force sensitivity to Ca2+. Estrogen binds to plasma membrane ER, leading to inhibition of agonist-activated mechanisms of VSM contraction. Possible nongenomic effects of estrogen include activation of K+ channels, leading to membrane hyperpolarization, inhibition of Ca2+ entry through Ca2+ channels, and thereby inhibition of Ca2+-dependent MLC phosphorylation and VSM contraction. Estrogen may also inhibit PKC and/or the MAPK pathway through activation of plasma membrane ERs and thereby further inhibit VSM contraction. Estrogen-induced NO release from the endothelium activates guanylate cyclase in VSM leading to increased cGMP and stimulation of cGMP-dependent protein kinase (PKG). PKG decreases [Ca2+]i by stimulating Ca2+ extrusion pumps in the plasma membrane and Ca2+ uptake pumps in SR and/or decrease the sensitivity of the contractile myofilaments to [Ca2+]i and thereby promote VSM relaxation. Estrogen also induces the release of PGI2 from the endothelium to activate the PGI2-cAMP pathway, or EDHF to activate Ca2+-activated K+ channels and to cause hyperpolarization and relaxation of VSM.
