Abstract
Abdominal tuberculosis is not a rare disease, but obstructive jaundice caused by tuberculosis (tuberculous lymphadenitis, tuberculous enlargement of the head of pancreas, and/or tuberculous stricture of the biliary tree) is rare. We recently experienced a case of obstructive jaundice as a result of paradoxical reaction of periportal tuberculous lymphadenopathy that was treated successfully with corticosteroid and biliary drainage. No similar cases have been reported previously.
Keywords: Paradoxical reaction, Periportal lymphadenitis, Abdominal tuberculosis
INTRODUCTION
The paradoxical reaction (PR) in antituberculosis therapy is defined as the clinical or radiological deterioration of pre-existing lesions or new lesion formation in patients who were improved with initial treatment.1 Most reported cases have complicated the treatment of cervical lymph node or cerebral disease and rarely reported in the abdominal tuberculosis.2-4 However, there is no reported case of obstructive jaundice as a result of PR of periportal tubercular lymphadenopathy. Therefore we report a case with biliary obstruction by enlarged lymph node which was successfully treated with corticosteroid and transient placement of a stent.
CASE REPORT
A 23-year-old man visited our hospital due to chronic diarrhea and weight loss. He has no history of injection drug abuse or homosexual activity. The antibody test for human immunodeficiency virus was negative. Abdominal computerized tomography (CT) scan showed diffuse reticularly-increased fat attenuation on the omentum, peritoneal thickening and multiple heterogeneously enhancing lymph nodes in portocaval, mesenteric root and ileocolic chain. The distal ileum and proximal ascending colonic walls were mildly thickened. The colonoscopic examination revealed transverse ulcers on the ileocecal valve and ascending colon (Fig. 1). The endoscopic biopsy demonstrated granulomatous inflammation (Fig. 2). Though the results of tuberculosis polymerase chain reaction (PCR) and acid fast bacilli (AFB) stain were negative, empirical antituberculous chemotherapy including isoniazide, rifampin, ethambutol, and pyrazinamide was started under the tentative diagnosis of tuberculosis colitis, peritonitis and lymphadenitis.
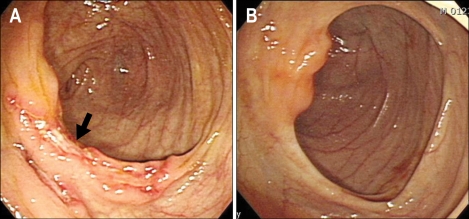
Fig. 1.
Colonoscopic examination revealed a transverse ulcer on the proximal portion of the ascending colon (A, arrow). This lesion disappeared after antituberculosis treatment (B).
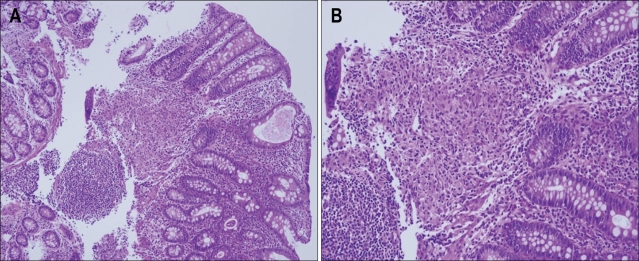
Fig. 2.
The pathology results of the endoscopic biopsy showing granulomatous inflammation (A, H&E stain, ×40: B, ×100).
Three months later, the second colonoscopic examination showed whitish ulcer scar on the ileocecal valve and newly developed protruding mass lesion with central shallow ulcer on the ascending colon (Fig. 3A, B). The follow-up abdominal CT revealed improvement of lymphadenopathies in the portocaval, mesenteric root and ileocolic chain. However, lymph node enlargements showing necrotic foci located at the periportal area were aggravated and about 2 cm sized newly enhancing mass was newly noticed in the ascending colon (Fig. 4B). The biopsy from newly developed mass demonstrated granuloma with negative AFB stain and negative tuberculosis PCR result. Because the clinical symptoms and the initial endoscopic finding were much improved with antituberculosis medication, we considered those changes as tuberculoma related to PR in antituberculosis treatment. Hence, oral prednisolone (30 mg/d) was added for 1 month and tapered. However, the patient was re-admitted due to epigastric pain and jaundice two weeks after the cessation of the corticosteroid therapy. The laboratory results were as follows: aspartate transaminase 171 IU/L (1-39 IU/L), alanine tranaminase 181 IU/L (1-39 IU/L), Alkaline phophatase 249 IU/L (25-100 IU/L), and total bilirubin 2.7 mg/dL (0.1-1.2 mg/dL). Follow-up CT scan revealed more enlarged necrotic lymphadenopathy at the periportal area and dilatation of the common bile duct (Fig. 4A). Endoscopic retrograde cholangiopancreatography (ERCP) was performed and an extrinsic compression of mid portion of the common bile duct by the extrinsic mass lesion was demonstrated (Fig. 5A). For reliving the obstruction, the patient underwent the endoscopic retrograde biliary drainage with a single 7 cm-sized 10 Fr polyethylene stent with a total length of 7 cm and additional oral corticosteroid therapy was re-started for 1 month and tapered (Fig. 5B). Six months later, he eventually recovered with antituberculosis medication. Follow-up colonoscopic examination and CT scan showed disappearance of previous lesions (Fig. 3B, C). Because the follow up ERCP revealed marked improvement of the previously obstructed biliary tree, the previously inserted common bile duct stent was removed. The cholangiogram after removal of the stent was nearly normalized (Fig. 5C).
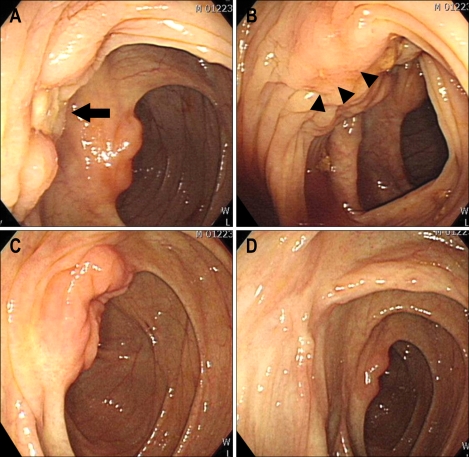
Fig. 3.
The second colonoscopic examination revealed white ulcer scar changes from the previous ulcerative lesion (A, arrow). However, a new protruding mass with a central ulcer was noted on the proximal ascending colon (B, arrowheads). Six months later, the ulcer lesion near the ileocecal valve was completely healed (C) and there were only white ulcer scar changes on the ascending colon (D).
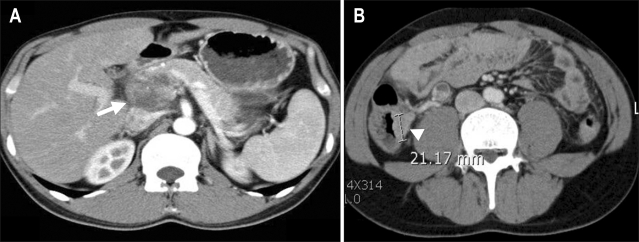
Fig. 4.
An enlarged lymph node was found in the periportal area (A, arrow). A newly developed, enhancing 2-cm mass lesion was noted on the proximal ascending colon (B, arrowhead).
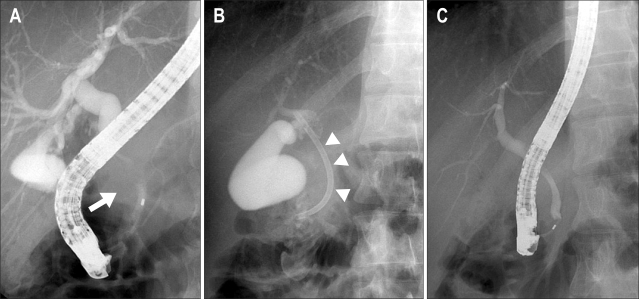
Fig. 5.
Endoscopic retrograde cholangiopancreatography revealed an extrinsic compression of the midportion of the common bile duct by the extrinsic mass (A, arrow). A 7-cm polyethylene stent was inserted successfully (B, arrowheads). The previously narrowed common bile duct was improved after the treatment (C).
DISCUSSION
PR during antituberculosis therapy is not a rare phenomenon.2,3 It is identified in 6-25% of patients receiving antituberculosis therapy.2-6 This phenomenon usually involves new or increasing enlargement of peripheral lymph nodes, cerebral tuberculomas, pulmonary infiltrates, or pleural disease. However, it is rare in the abdominal tuberculosis.2-4 PRs most frequently occur during the first few months of antituberculosis treatment and usually resolve with continued therapy.3,7 Biopsies of affected tissues usually show granulomatous inflammation with negative AFB smears and cultures.8
The mechanisms of PR have been understood as decrease in suppressor mechanisms related to active tuberculosis infection, strengthening of delayed hypersensitivity response of the host, and heavy exposure to mycobacterial antigens following bactericidal tuberculosis chemotherapy.7,9 Cheng et al.3 reported that the risk factors for development of PR during antituberculosis therapy in HIV-negative patients are extra-pulmonary tuberculosis, with or without pulmonary involvement and lower baseline lymphocyte counts.
However, PR during antituberculosis therapy remains a diagnostic dilemma. The diagnosis can only be confirmed after exclusion of other differential diagnosis such as secondary infections, inadequate therapy or drug resistance, poor compliance and a side effect of antituberculosis therapy. In our case, we considered the aggravated lymphadenopathies and newly developed mass lesion on the ascending colon as PR because his clinical symptoms were disappeared and other lesions found on the second colonoscopic examination were improved with antituberculosis medications. Hence, the antituberculosis therapy was continued and additional corticosteroid treatment was tried. The obstruction of the common bile duct by the enlarged periportal lymph node was successfully treated with additional corticosteroid therapy and biliary stenting.
While PRs are usually self-limited, respiratory failure and death can be occurred.10 Sometimes corticosteroid is used for relieving PR. The known advantage of corticosteroid therapy is reducing edema around enlarging intracranial tuberculomas. However, it is unclear that the advantage of steroid therapy for lymph node tuberculosis.7 Some reported rapid recovery after the initiation of corticosteroid therapy.5,11-13 The treatment resulted in clinical improvement in many cases in a median time of 3 days. Effective doses of the prednisolone ranged from 10 to 80 mg daily. When corticosteroids were tapered gradually, PR recurred in one third of the patients after their discontinuation.2,3 Most patients with relapsed PR responed again to corticosteroid retreatment.2 However, in other reports, continued deteriorations have been reported despite of corticosteroid treatment.12 Furthermore, PRs have occurred in patients who were given corticosteroid from the outset of their antituberculosis treatment and another report has provided PRs that resolved without corticosteroid treatment.14,15
Obstructive jaundice caused by tuberculosis is rare. It can be caused by tuberculous lymphadenitis,16 tuberculosis of the pancreatic head,17 biliary stricture after biliary tuberculosis18 or lymph node fistulation in the common bile duct.19 There were no reported cases of obstructive jaundice related with tuberculous lymphadenitis, especially during antituberculosis therapy. In addition, most cases of periportal lymphadenitis were confirmed by diagnostic laparoscopy or surgical resection.17,20 The mainstay of treatment in bile duct stricture or extrinsic compression by tuberculous lesion is to relieve the obstruction and to administer antituberculosis therapy. Surgical bypass for the relief of bile duct obstruction is often required.21 Endoscopic stenting of the stricture has also been reported.18,21,22 In our case, corticosteroid therapy and biliary stent for 6 months successfully reduced the extrinsic compression by the enlarged lymph node. To our knowledge, there was no reported case of common bile duct obstruction by lymphadenopathy related to PR during antituberculosis therapy, which was treated by endoscopic stenting and corticosteroid.
We emphasize that the worsening of tuberculous lesions may occur during antituberculosis chemotherapy even in case of the intestinal tuberculosis and does not necessarily indicate treatment failure. The combination of steroid therapy and biliary stenting can be an option in the treatment of obstructive jaundice by PR of periportal lymphadenitis.
References
- 1.Chambers ST, Hendrickse WA, Record C, Rudge P, Smith H. Paradoxical expansion of intracranial tuberculomas during chemotherapy. Lancet. 1984;2:181–184. doi: 10.1016/s0140-6736(84)90478-1. [DOI] [PubMed] [Google Scholar]
- 2.Breen RA, Smith CJ, Bettinson H, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59:704–707. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng VC, Ho PL, Lee RA, et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002;21:803–809. doi: 10.1007/s10096-002-0821-2. [DOI] [PubMed] [Google Scholar]
- 4.Cheng VC, Yam WC, Woo PC, et al. Risk factors for development of paradoxical response during antituberculosis therapy in HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2003;22:597–602. doi: 10.1007/s10096-003-0998-z. [DOI] [PubMed] [Google Scholar]
- 5.Al-Majed SA. Study of paradoxical response to chemotherapy in tuberculous pleural effusion. Respir Med. 1996;90:211–214. doi: 10.1016/s0954-6111(96)90289-9. [DOI] [PubMed] [Google Scholar]
- 6.Memish ZA, Mah MW, Mahmood SA, Bannatyne RM, Khan MY. Clinico-diagnostic experience with tuberculous lymphadenitis in Saudi Arabia. Clin Microbiol Infect. 2000;6:137–141. doi: 10.1046/j.1469-0691.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 7.Hawkey CR, Yap T, Pereira J, et al. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis. 2005;40:1368–1371. doi: 10.1086/429317. [DOI] [PubMed] [Google Scholar]
- 8.Chien JW, Johnson JL. Paradoxical reactions in HIV and pulmonary TB. Chest. 1998;114:933–936. doi: 10.1378/chest.114.3.933. [DOI] [PubMed] [Google Scholar]
- 9.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 10.Onwubalili JK, Scott GM, Smith H. Acute respiratory distress related to chemotherapy of advanced pulmonary tuberculosis: a study of two cases and review of the literature. Q J Med. 1986;59:599–610. [PubMed] [Google Scholar]
- 11.Hejazi N, Hassler W. Multiple intracranial tuberculomas with atypical response to tuberculostatic chemotherapy: literature review and a case report. Infection. 1997;25:233–239. doi: 10.1007/BF01713151. [DOI] [PubMed] [Google Scholar]
- 12.Lees AJ, MacLeod AF, Marshall J. Cerebral tuberculomas developing during treatment of tuberculous meningitis. Lancet. 1980;1:1208–1211. doi: 10.1016/s0140-6736(80)91676-1. [DOI] [PubMed] [Google Scholar]
- 13.Place S, Knoop C, Remmelink M, et al. Paradoxical worsening of tuberculosis in a heart-lung transplant recipient. Transpl Infect Dis. 2007;9:219–224. doi: 10.1111/j.1399-3062.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Afghani B, Lieberman JM. Paradoxical enlargement or development of intracranial tuberculomas during therapy: case report and review. Clin Infect Dis. 1994;19:1092–1099. doi: 10.1093/clinids/19.6.1092. [DOI] [PubMed] [Google Scholar]
- 15.Carter EJ, Mates S. Sudden enlargement of a deep cervical lymph node during and after treatment for pulmonary tuberculosis. Chest. 1994;106:1896–1898. doi: 10.1378/chest.106.6.1896. [DOI] [PubMed] [Google Scholar]
- 16.Obama K, Kanai M, Taki Y, Nakamoto Y, Takabayashi A. Tuberculous lymphadenitis as a cause of obstructive jaundice: report of a case. Surg Today. 2003;33:229–231. doi: 10.1007/s005950300051. [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Yang CC, Yeh YH, Yang JC, Chou DA. Pancreatic tuberculosis with obstructive jaundice--a case report. Am J Gastroenterol. 1999;94:2534–2536. doi: 10.1111/j.1572-0241.1999.01389.x. [DOI] [PubMed] [Google Scholar]
- 18.Fan ST, Ng IO, Choi TK, Lai EC. Tuberculosis of the bile duct: a rare cause of biliary stricture. Am J Gastroenterol. 1989;84:413–414. [PubMed] [Google Scholar]
- 19.Colovic R, Grubor N, Jesic R, et al. Tuberculous lymphadenitis as a cause of obstructive jaundice: a case report and literature review. World J Gastroenterol. 2008;14:3098–3100. doi: 10.3748/wjg.14.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosaka A, Masaki Y, Yamasaki K, Aoki F. Isolated periportal tuberculosis: characteristic findings of clinical imaging. J Gastrointest Surg. 2008;12:779–781. doi: 10.1007/s11605-007-0306-9. [DOI] [PubMed] [Google Scholar]
- 21.Kok KY, Yapp SK. Tuberculosis of the bile duct: a rare cause of obstructive jaundice. J Clin Gastroenterol. 1999;29:161–164. doi: 10.1097/00004836-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Bearer EA, Savides TJ, McCutchan JA. Endoscopic diagnosis and management of hepatobiliary tuberculosis. Am J Gastroenterol. 1996;91:2602–2604. [PubMed] [Google Scholar]