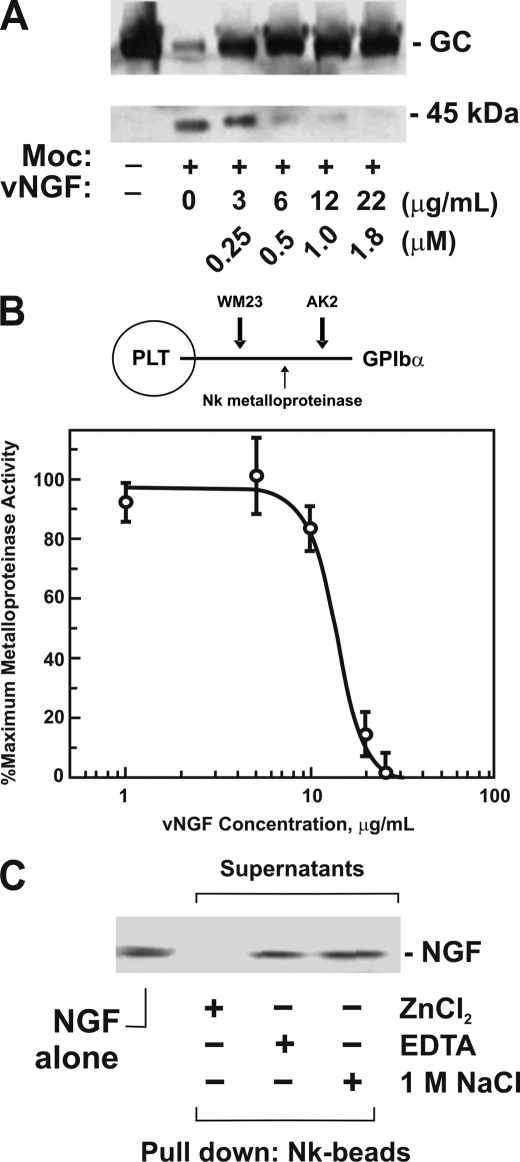
FIGURE 3.
Effect of NGF on snake venom metalloproteinase activity. A, venom NGF inhibits mocarhagin-dependent digestion of glycocalicin. Glycocalicin (GC; ∼30 μg/ml, final concentration) was treated with mocarhagin (Moc; 5 μg/ml or ∼0.1 μm, final concentration) in TS buffer containing 1 mm ZnCl2, 1 mm CaCl2 for 60 min at 22 °C in the absence or presence of venom NGF (vNGF, 0–22 μg/ml or 0–1.8 μm for NGF monomer, final concentration). Samples were analyzed on SDS-polyacrylamide gels (reducing conditions) and blotted with rabbit anti-glycocalicin IgG visualized using horseradish peroxidase-conjugated secondary antibody and ECL. B, venom NGF blocks Nk-dependent inhibition of GPIbα-dependent platelet (PLT) adhesion to AK2. Washed platelets (1 × 108/ml) were treated with Nk metalloproteinase (5 μg/ml, final concentration) in the absence or presence of venom NGF (0–40 μg/ml) for 60 min at 22 °C. Platelet adhesion to microtiter wells coated with anti-GPIbα monoclonal antibody, AK2 (with epitope lost following Nk treatment, upper diagram), was measured using anti-glycocalicin IgG as described under “Experimental Procedures.” Nk-dependent cleavage was confirmed by measuring platelet adhesion to WM23-coated wells, where the epitope is unaffected by Nk (27). The error bars represent 1× S.D. C, binding of venom NGF to Nk-coated beads. Purified venom NGF (5 μg/ml, final concentration) incubated with Nk-agarose beads (25% (v/v) suspension) in TS buffer for 30 min at 22 °C in the presence of EDTA, 1 mm ZnCl2, 1 mm CaCl2, or 1 m NaCl was analyzed after pelleting the resin by centrifugation and SDS-polyacrylamide gel electrophoresis and Coomassie Blue staining.