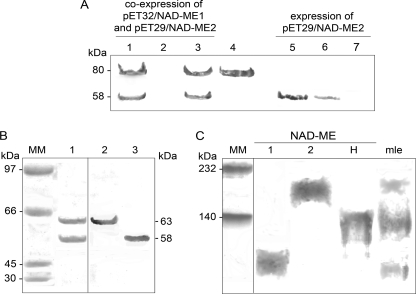
FIGURE 1.
Purification of recombinant NAD-MEH by co-expression of NAD-ME1 and NAD-ME2. A, purification steps from E. coli BL21 cells co-transformed with pET32-NAD-ME1 and pET29-NAD-ME2 (lanes 1–3) or E. coli BL21 cells transformed with pET29-NAD-ME2 (lanes 5–7) were analyzed by Western blot after SDS-PAGE using a mixture of antibodies against NAD-ME1 and NAD-ME2. Lanes 1 and 5, 20 μg of E. coli crude extract after induction; lanes 2 and 6, last nickel-nitrilotriacetic acid column fraction wash; lanes 3 and 7, elute fraction with 200 mm imidazole. Purified NAD-ME1 fusion protein (3 μg) was loaded in lane 4. B, shown is Coomassie Blue-stained SDS-PAGE (lane 1, 10 μg) of purified NAD-MEH after enterokinase digestion. The same protein was analyzed by Western blot using antibodies against NAD-ME1 (Lane 2, 2 μg) or against NAD-ME2 (Lane 3, 2 μg). MM, molecular weight marker. C, shown is a Western blot analysis after native-PAGE of recombinant NAD-MEs using a mixture of antibodies against NAD-ME1 and NAD-ME2. Approximately 5 μg of NAD-ME1, -2, and -H were loaded. A mitochondrial leaf crude extract (mle, 30 μg) was also loaded on the gel. Molecular weight markers (MM) were run in parallel and stained with Coomassie blue.