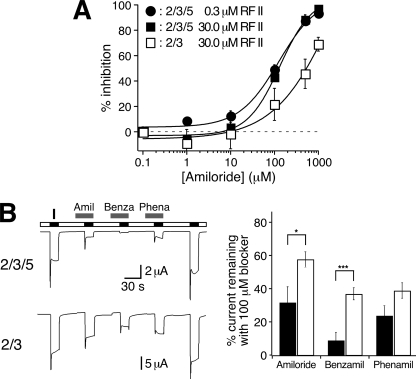
FIGURE 7.
HyNaC5 increases the apparent affinity for amiloride and amiloride analogs. A, concentration response curve for the inhibition of HyNaC currents by amiloride. Curves were determined with 0.3 μm (HyNaC2/3/5) and 30 μm Hydra-RFamide II (HyNaC2/3 and HyNaC2/3/5), respectively (n = five oocytes for HyNaC2/3 and n = 6 oocytes for HyNaC2/3/5). B, left, representative current traces of whole oocytes either expressing HyNaC2 and -3 or HyNaC2, -3, and -5. Channels were activated in the presence of different blockers (100 μm). Right, bar graphs illustrating the current remaining in the presence of the respective blockers; black bars represent HyNaC2/3/5, white bars HyNaC2/3. Before and after application of the blockers, channels were activated without blockers; the mean value of these two measurements was used to normalize the currents obtained in the presence of the blockers. Error bars represent S.E. (six oocytes); HyNaCs were activated with 30 μm (HyNaC2/3) or 1 μm Hydra-RFamide I (HyNaC2/3/5), respectively. In the presence of HyNaC5, amiloride (Aml) and benzamil (Benza) blocked significantly more current, suggesting an increased affinity for these blockers. *, p < 0.05; ***, p < 0.001, two-tailed t test. Phena, phenamil.