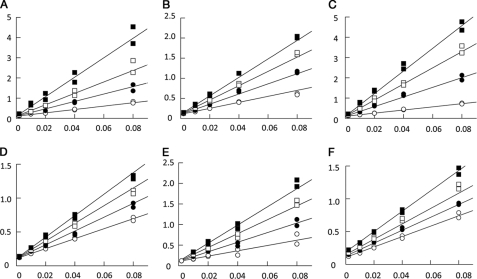
FIGURE 4.
Competitive inhibition of aldolase by LC4 and WASP peptides. Aldolase shows reciprocal reaction velocity (1/velocity in mg/unit) when plotted against the reciprocal substrate concentration (1/[FPB] in μm−1) for the cleavage of FBP in the presence of 7- and 20-mer LC4 peptides of SNX9. Duplicates are shown. A, 0 (open circle), 5 (closed circle), 10 (open square), and 20 μm (closed square) 7-mer peptide (LC4 residues 165–171); B, 0 (open circle), 100 (closed circle), 200 (open square), and 300 μm (closed square) 7-mer peptide except W165A; C, 0 (closed circle), 35 (closed circle), 70 (open square), and 105 μm (closed square) 20-mer peptide (LC4 residues 163–183); D, 0 (open circle), 50 (closed circle), 100 (open square), and 150 μm (closed square) 20-mer W165A mutated peptide; E, 0 (open circle), 431 (closed circle), 862 (open square), and 1293 μm (closed square) 7-mer peptide (WASP residues 496–502); and F, 0 (open circle), 363 (closed circle), 726 (open square), and 1452 μm (closed square) 7-mer random sequence peptide (DNYEFDG) as a negative control. Inhibition kinetics was performed using crystallization buffer conditions.