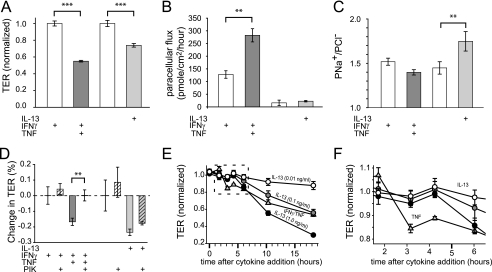
FIGURE 2.
IL-13 and TNF cause barrier dysfunction by different mechanisms. A, TNF (dark gray bar; 2.5 ng/ml) and IL-13 (light gray bar; 0.1 ng/ml) reduced TER of T84 monolayers cells at 4 and 12 h, respectively. Prior to TNF treatment, monolayers were cultured with IFNγ (white bar; 2.5 ng/ml) for 24 h to induce TNF receptor expression, which also reduced TER. TER was normalized to untreated controls (3.06 ± 0.22 kΩ cm2) for IL-13 or IFNγ-treated controls (1.83 ± 0.02 kΩ cm2) for TNF-induced changes. B, TNF (dark gray bar), but not IL-13 (light gray bar), increased the paracellular permeability of 4-kDa dextran. Note that IFNγ pretreatment (white bar) alone also caused an increase in 4-kDa dextran permeability, consistent with the observed effect on TER. C, IL-13 (light gray bar), but not TNF (dark gray bar), increased PNa+/PCl− of T84 monolayers. D, the specific MLCK inhibitor PIK (all hatched bars; 330 μm) corrected barrier dysfunction after TNF (dark gray hatched bars), but not IL-13 (light gray hatched bars), treatments. E, barrier dysfunction induced by TNF (triangles; 2.5 ng/ml) developed more rapidly than after IL-13 treatment (circles; 0.1, 1, or 10 ng/ml, as indicated). F, data for the first 5 h after cytokine treatment (from E) demonstrate a dose-independent delay in TER loss induced by IL-13 relative to TNF. IL-13-induced barrier dysfunction occurred between 4 and 6 h, whereas TNF-induced barrier dysfunction was first apparent between 2 and 3 h after initiation of cytokine treatment (**, p ≤ 0.01; ***, p ≤ 0.001, S.E.).