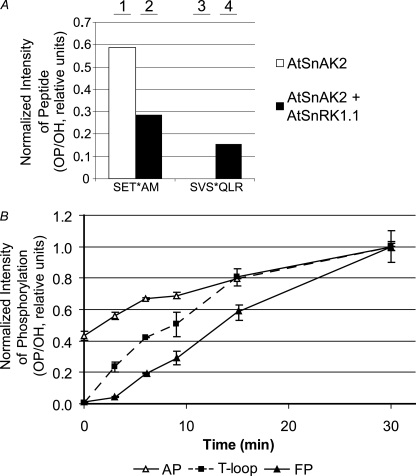
FIGURE 4.
MS/MS analysis of AtSnAK2 and AtSnRK1.1 phosphorylation sites. A, proteins (20 μg of AtSnAK2 and 40 μg of AtSnRK1.1 preparations) were incubated in kinase activity buffer (containing 1 mm ATP) at 30 °C for 60 min and then submitted to SDS-PAGE. Ratios of phosphopeptides to unmodified peptides (OP/OH) from the corresponding protein bands were determined by MS/MS. SET*AM and SVS*QLR (asterisk marking the phosphoresidue) were the two phosphorylated sites found on AtSnAK2. B, time course of the phosphopeptide to unmodified peptide ratio (OP/OH) for each phosphorylation site normalized to the maximum value of each reaction (after 30 min). Proteins (10 μg of each preparation) were prepared as described in A. Corresponding bands were analyzed by MS/MS and peptides containing the T-loop site of AtSnRK1.1 (squares), AP (SET*AM, open triangles), and FP (SVS*QLR, closed triangles) sites of AtSnAK2 were quantified. Results are the average of three independent experiments ± S.D. (error bars).