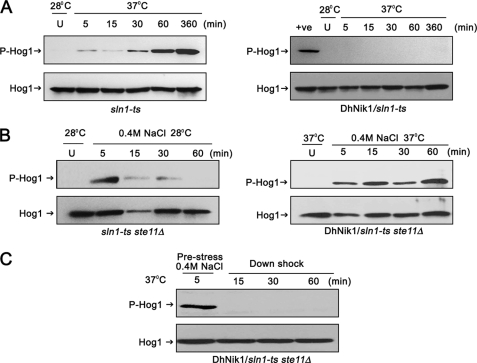
FIGURE 2.
Regulation of HOG pathway in S. cerevisiae by DhNIK1. A, immunoblots showing the level of Hog1p phosphorylation in S. cerevisiae strain TM229 (sln1-ts) or TM229 expressing DhNik1p at nonpermissive temperature (37 °C). Cells were grown on SD medium with the required nutrient supplement at 28 °C until exponential phase (A600 ∼0.7) before exposing them to 37 °C for indicated time. The level of dually phosphorylated Hog1p (P-Hog1) was detected by immunoblotting the total cell extracts with anti-phospho-p38 antibody (Cell Signaling Inc). The total extract from cells grown at 28 °C was loaded as control (U). The total cell extracts from TM229 exposed to 37 °C for 1 h are included in the right panel as positive control (+ve). Blots were re-probed with anti Hog1 antibody (Y215; Santa Cruz Biotechnology) to detect total Hog1. P-Hog1 or Hog1 indicate phosphorylated or total Hog1p in the blot. B, immunoblots showing high osmolarity-induced Hog1p phosphorylation in S. cerevisiae strain NM2 (sln1-ts ste11Δ) at 28 °C (left panel) and NM2 expressing DhNik1p at 37 °C (right panel). Cells at A600 ∼0.7 were exposed to 0.4 m NaCl for different lengths of time as indicated, and total cell extract from these samples was used for blotting. Total cell extracts from respective cultures not exposed to osmotic stress were loaded as control (U). C, level of dually phosphorylated Hog1p after down-shifting to low osmolarity. NM2 carrying DhNIK1 was grown at 28 °C up to A600 ∼0.7. Cells were incubated at 37 °C for another hour before exposing to 0.4 m NaCl for 5 min at 37 °C. Following centrifugation at 37 °C (to prevent the reactivation of sln1-ts), the cell pellet was resuspended in SD media without salt (maintained at 37 °C) and incubated further at 37 °C for different times as indicated. Total protein extract made from these samples were immunoblotted. All the blots shown above are representative of at least three different experiments.