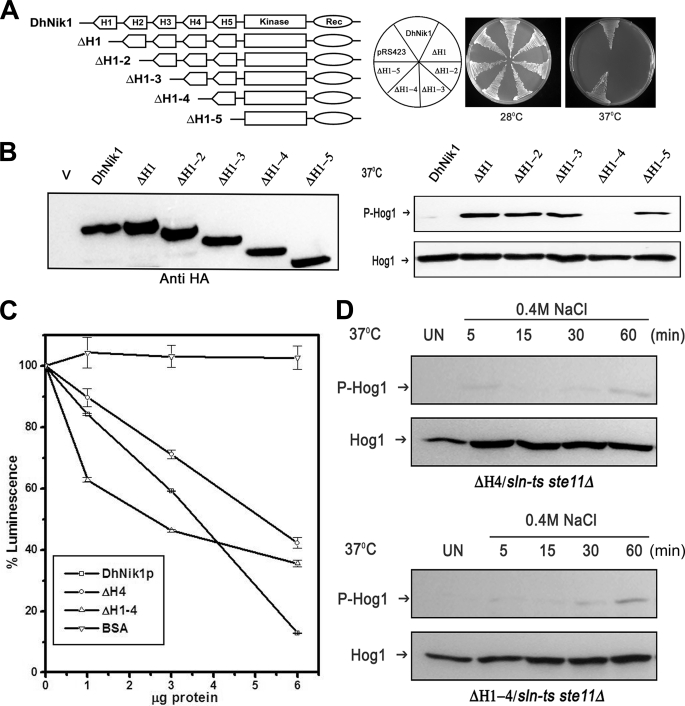
FIGURE 4.
Effect of serial deletions of HAMP domains in DhNIK1. A, diagrammatic representations of serially deleted HAMP domain mutants and growth patterns of TM229 carrying vector pRS423, DhNik1, or different HAMP domain deleted mutants on minimal SD agar plate without histidine at 28 or 37 °C are shown. Experiments were repeated three times with similar results. B, left panel, immunoblot showing the level of expression of DhNik1p, HAMP-deleted mutants ΔH1, ΔH1–2, ΔH1–3, ΔH1–4, and ΔH1–5 after expressing them as HA-tagged proteins. Total cell extract from TM229 transformed with pRS423 were loaded as control (V). Right panel, immunoblots showing the phosphorylated Hog1p in cells with different HAMP domain deletion mutants. S. cerevisiae strain TM229 (sln1-ts) carrying DhNik1 or different mutants (ΔH1, ΔH1–2, ΔH1–3, ΔH1–4, and ΔH1–5) were grown on SD minimal media without histidine at 28 °C until A600 ∼0.7 and exposed to 37 °C for 1 h. The level of phosphorylated Hog1p in these cells was detected by immunoblotting as described previously. Experiments were repeated three times with similar results. C, kinase activities of DhNik1p, ΔH4, and ΔH1–4 were measured by a luminescence assay. Assay was done without any protein or with 1, 3, and 6 μg of purified, recombinant proteins (DhNik1p, ΔH4, and ΔH1–4). Decreasing luminescence indicated increasing kinase activity. Bovine serum albumin (BSA) was used as a negative control. The luminescence observed with different protein concentrations was expressed as percentage of that obtained with the corresponding reaction without any protein. Data are the means ± S.D. of three experiments. D, high osmolarity induced Hog1p phosphorylation in S. cerevisiae strain NM2 (sln1-ts ste11Δ) expressing HAMP domain deletion mutant ΔH4 and mutant ΔH1–4. Cells were grown on SD minimal media without histidine at 28 °C until A600 ∼0.7 and exposed to 37 °C for 1 h. Osmotic stress was given to these cells by exposing them to 0.4 m NaCl at 37 °C for different lengths of time as indicated, and total cell extract from these samples was used for blotting. Total cell extract from respective culture not exposed to osmostress (Un) was loaded as control. Blots were re-probed with anti Hog1 antibody. P-Hog1 or Hog1 indicate phosphorylated or total Hog1p in the blot.