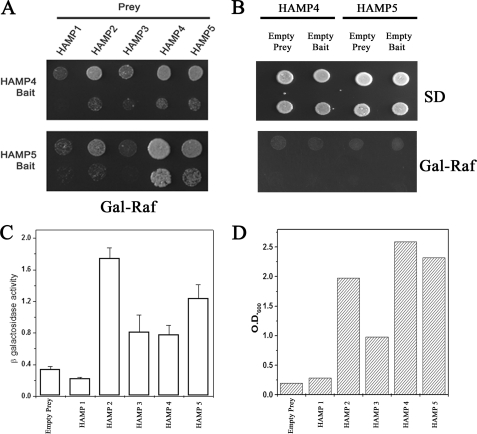
FIGURE 5.
Interactions between HAMP domains of DhNik1p. A, two-hybrid assay for interactions between HAMP domains. HAMP4 or HAMP5 cloned in pEG202 vector was used as bait, and five HAMP domains were cloned individually in plasmid pJG4-5 for prey construction. Both bait and prey constructs (in pairs as indicated) were transformed into S. cerevisiae strain EGY48. Growth of the transformants on Gal-Raf minimal medium is shown after dilution spotting. Results are representative of three different experiments. B, two-hybrid interactions in HAMP4 and HAMP5 with empty bait or prey combinations. S. cerevisiae strain EGY48 transformed with HAMP4 bait/empty prey; HAMP4 prey/empty bait; HAMP5 bait/empty prey, and HAMP5 prey/empty bait were grown in minimal SD medium, and serial dilution of the culture was spotted onto minimal SD and Gal-Raf plate. C, β-galactosidase activity in yeast strain EGY48 harboring lacZ reporter plasmid, HAMP4 bait along with empty prey or different HAMP domains in prey vector. Experiments were repeated with two independent pools of six transformants each. β-Galactosidase activity is expressed as nanomoles of o-nitrophenyl β-d-galactopyranoside utilized per min by 1 ml of culture after normalizing its A600 to 1.0. D, EGY48 harboring HAMP4 bait along with empty prey or different HAMP domains in prey vector were grown overnight in minimal media with 2% raffinose (without tryptophan and histidine). The cultures were re-inoculated in minimal media with 1% raffinose and 2% galactose (without tryptophan, histidine and leucine) at A600 ∼0.10 and grown further for 40 h. Representative data of two independent experiments are shown here.