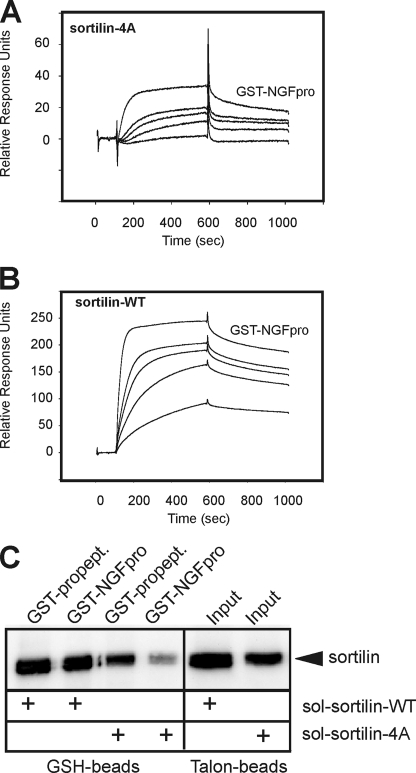
FIGURE 7.
GST-NGFpro binding to sortilin-WT and sortilin-4A. SPR analysis showing concentration series of GST-NGFpro (10, 20, 30, 40, and 50 nm) tested for binding to immobilized extracellular domains of sortilin-4A (A) and sortilin-WT (B), demonstrating a strong decrease in the binding capacity for the NGF pro-domain upon the quadruple mutation in sortilin-4A. A higher than 10-fold decrease in affinity (WT: KD ∼2 nm versus 4A: KD ∼26 nm) was estimated using the BIAevaluation software. C, medium from EBNA 293 cells producing either the soluble ectodomain of sortilin-WT or sortilin-4A were incubated with a GST-tagged variant of the receptor propeptide (GST-propept) or GST-NGFpro and precipitated by glutathione (GSH) beads. The amount of total secreted sortilin-WT and sortilin-4A (i.e. input) was determined by precipitation using Talon beads binding to the histidine tag within the sortilin domains. The precipitated proteins were subjected to SDS-PAGE analysis and visualized by Western blot analysis for sortilin.