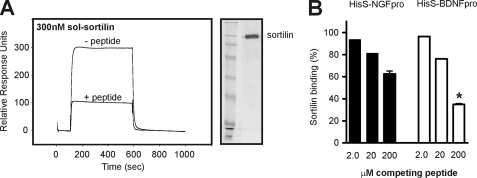
FIGURE 9.
Sortilin-derived peptide specifically competes binding of pro-NT to sortilin. A, recombinant sortilin was purified from 293 cells (indicated to the right by silver-stained SDS-PAGE analysis), and used for SPR studies to immobilized HisS-BDNFpro. The signal of 300 RU observed for binding in the absence of the peptide sort166–181 (− peptide) was significantly lowered to 100 RU in the presence of 200 μm linear sortilin antagonist (+ peptide). B, sortilin binding to either HisS-NGFpro or HisS-BDNFpro was determined by SRP analysis (as exemplified in A) and the inhibition by increasing amounts of sort166–181 (at 2, 20, and 200 μm) was plotted relative to the observed interaction in the absence of competitor (in percentage). Values represent the mean ± S.E. from three experiments. *, p = 0.0007; two-tailed Student's t test.