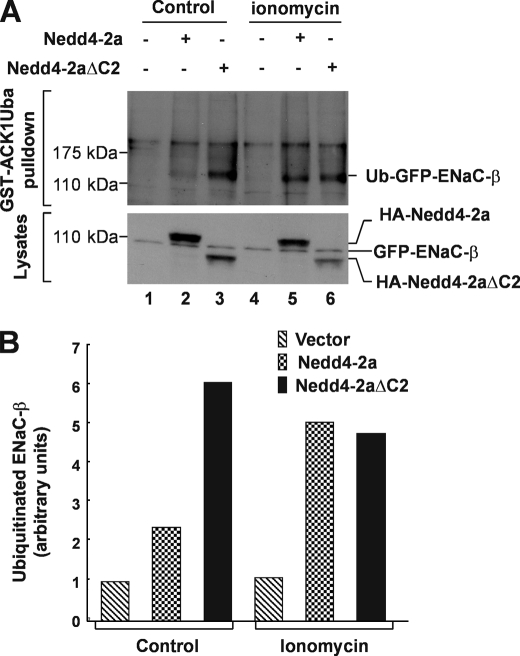
FIGURE 4.
Influx of extracellular calcium by ionomycin enhances ubiquitination of ENaC-β subunit, a specific Nedd4 substrate. A, a stably GFP-ENaC-β-expressed HEK293 cells transfected 36 h with HA-Nedd4-2a, Nedd4-2aΔC2, or vector were serum-starved for 12 h followed by incubation in calcium buffer (20 mm Hepes, pH 7.4, 140 mm NaCl, 6 mm KCl, 1 mm MgCl2, 1.25 mm CaCl2, 0.1 mm EDTA, and 20 mm glucose) along with or without 1 μm ionomycin for 6 h in presence of 10 μm MG-132, a proteasome inhibitor. The ubiquitinated GFP-ENaC-β was precipitated by GST-ACK1-Uba and detected by immunoblotting with anti-GFP antibody (top panel). The expression level of GFP-ENaC-β, HA-Nedd4-2a, or HA-Nedd4-2aΔC2 was determined by immunoblotting of the cell lysates with anti-GFP and anti-HA, respectively (bottom panel). B, the quantification of ubiquitinated GFP-ENaC-β in (A) with GE-Gel Logic100 Imaging system.