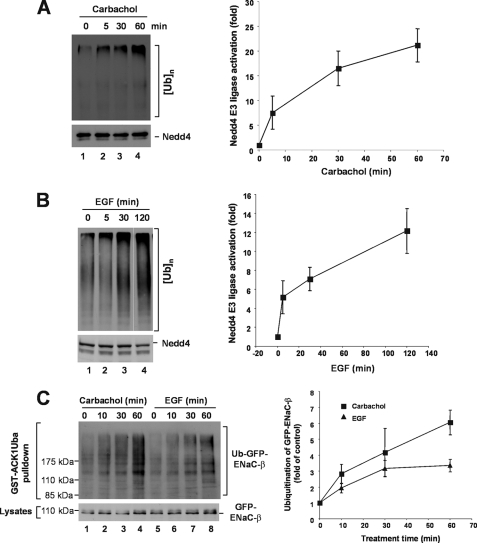
FIGURE 5.
In vivo activation of Nedd4 E3 ubiquitin ligase activity by stimulation of acetylcholine receptor and EGFR. A, HEK293 cells were starved in serum-free Dulbecco's modified Eagle's medium for 2 h, then stimulated with 5 mm carbachol at the indicated time. B, human breast cancer cell MDA-MB-231 cells were serum-starved overnight followed by stimulation with EGF (100 ng/ml) at indicated time. In both A and B, endogenous Nedd4 was immunoprecipitated with an anti-Nedd4 antibody from 20,000 × g cleared cell homogenates. The E3 ubiquitin ligase activity was assayed. The poly-ubiquitin products were determined by immunoblotting and quantified by using a Kodak EDAS290 imaging system for two independent experiments. C, stimulation of acetylcholine receptor by carbachol or EGFR by EGF enhances ubiquitination of Nedd4 substrate ENaC-β subunit. GFP-ENaC-β-expressed HEK293 cells were serum-starved for 12 h, then stimulated with 5 μm carbachol or 100 ng/ml EGF at the indicated times in the presence of 10 μm MG-132. The ubiquitinated GFP-ENaC-β was precipitated by GST-ACK1-Uba and detected by immunoblotting with anti-GFP (top panel). The expression level of GFP-ENaC-β was determined by immunoblotting of the cell lysates with anti-GFP (bottom panel). Ubiquitinated GFP-ENaC-β was quantified with normalized GFP-ENaC-β by using a GE-Gel Logic100 imaging system for two independent experiments.