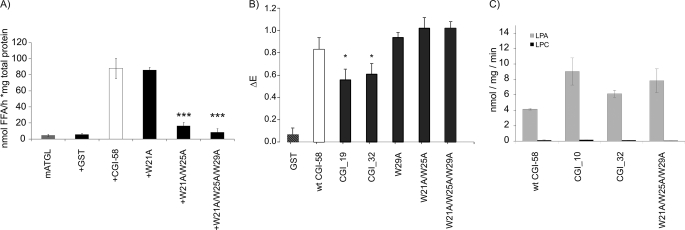
FIGURE 6.
Trp site directed mutations affect the capacity of CGI-58 to activate ATGL TG hydrolase activity but not to physically interact with ATGL. A, stimulation of mATGL TG hydrolase activity by cell lysates expressing mCGI-58 Trp mutations. The soluble fraction of Trp point mutations of mCGI-58 (GST-tagged, expressed in E. coli) were tested for their activation capacity on bacterially expressed mATGL using [3H]triolein as substrate. B, protein-protein interaction of ATGL with truncations and mutations of mCGI-58 were tested by ELISA. Plates were coated with mATGL and incubated with cell extracts containing GST-tagged proteins, and the amounts of retained CGI-58 were determined using CGI-58-specific antiserum. C, mCGI-58, N-terminal truncation mutants, and the triple mutant W21_25_29A were tested for acyltransferase activity using LPA (gray) or lysophosphatidylcholine (black) as substrates. Data are presented as mean ± S.D. (A and B) or mean ± S.E. (C); *, p < 0.05; ***, p < 0.001.