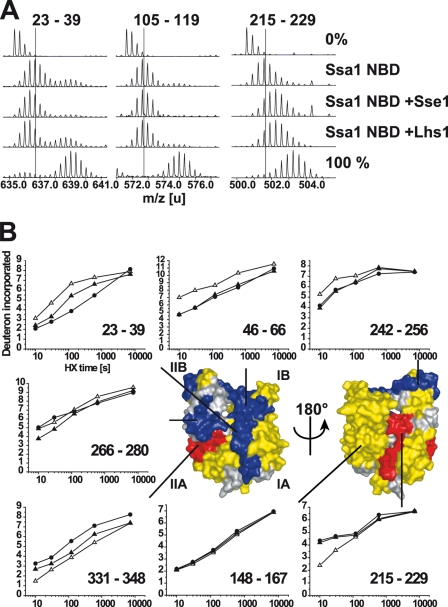
FIGURE 5.
HX properties of the Ssa1 NBD in complex with Lhs1. A, mass spectra of representative peptides from the monomeric Ssa1 NBD or Ssa1 NBD in complex with Lhs1 or Sse1 incubated in H2O (0%), or for 10 s in D2O buffer. Control spectra (100%) of the same peptides from fully deuterated Ssa1. The lines indicate the centroids of the peptide ions for monomeric Ssa1 NBD after 10 s in D2O buffer. B, kinetics of deuteron incorporation during HX into selected segments of the Ssa1 NBD in its monomeric form (▵) and when in complex with Lhs1 (▴) or Sse1 (●) from data set one (see supplemental Fig. S2, A and B). The structural representation of the Ssa1 NBD (modeled onto the structure of Hsc70 NBD (33)) is colored to display segments in which Lhs1 and Sse1 induce average protection/deprotection effects at 10 s, 2 min, and 10 min of ≥0.5 Da and 5% of total possible exchange compared with the monomeric NBD in duplicate data sets (see supplemental Fig. S2); HX protection (blue), deprotection (red), no significant HX differences (yellow), or no data (gray) are as indicated.