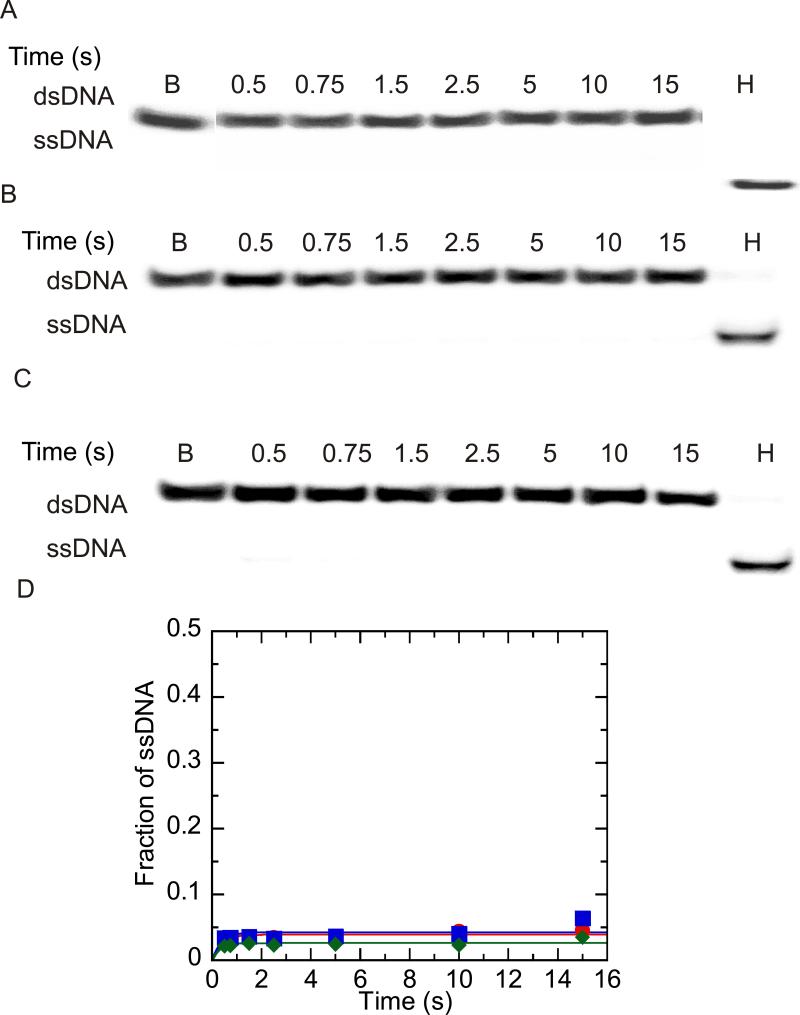
Figure 2.
Unwinding of 37:22-mer partial duplex by NS3 and NS3h under conditions of DNA substrate concentration in excess of enzyme concentration. Unwinding products were resolved by electrophoresis on a 20 % native polyacrylamide gel, visualized using a PhosphorImager and quantitated by using ImageQuant software. (A) Representative gel image showing unwinding of 200 nM partial duplex DNA by 100 nM NS3. The blank sample (B) shows the DNA substrate prior to unwinding, and the heated sample (H) show the substrate after heating to 95 °C. (B) Representative gel image showing unwinding of 200 nM partial duplex DNA by 100 nM NS3h. (C) Heparin (4 mg/ml) was mixed with the partial duplex substrate (200 nM) prior to initiating the reaction by mixing with NS3h (100 nM) to determine the ability of heparin to serve as a protein trap. (D) Fraction of ssDNA product formed after quantitation of gel images by using Imagequant software. Unwinding of 200 nM 37:22-mer DNA by 100 nM NS3 ( ), 100 nM NS3h (
), 100 nM NS3h ( ) and 500 nM NS3h under conditions where heparin and partial duplex DNA were mixed together prior to initiation of the reaction by addition of enzyme. (
) and 500 nM NS3h under conditions where heparin and partial duplex DNA were mixed together prior to initiation of the reaction by addition of enzyme. ( ).
).