TABLE 1.
Carboamination of γ-(N-Arylamino)alkenes with Aryl Chloridesa
| entry | amine | product | regioselectivityb | yieldc |
|---|---|---|---|---|
| 1 |
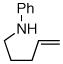 1a |
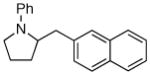 2a |
43:3:0:1 | 79% |
| 2 | 1a |
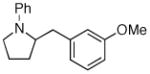 2b |
38:3:0:1 | 74% |
| 3 | 1a |
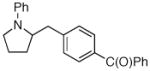 2c |
27:2:0:1 | 79% |
| 4 |
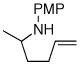 1b |
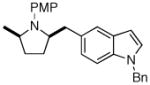 2d |
10:1:0:0 | 66% (>20:1 dr) |
| 5 |
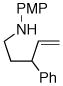 1c |
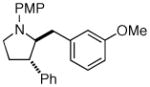 2e |
>20:1 | 65% (>20:1 dr)d |
| 6 |
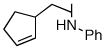 1d |
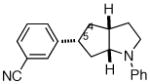 2f |
11:1e | 70% (>20:1 dr)f |
| 7 | 1d |
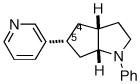 2g |
12:1e | 69% (>20:1 dr)f |
Conditions: amine (1.0 equiv), aryl chloride (1.1–1.4 equiv), NaOtBu (1.2 equiv), Pd2(dba)3 (1 mol %), PCy2Ph (4 mol %), toluene (0.25 M), 110 °C.
Determined by GC and GC/MS analysis. The minor regioisomers formed are analogous to 3–5 shown in eq 1.
Isolated yield (average of two or more experiments).
PCy3•HBF4 was used in place of PCy2Ph.
The minor regioisomer was arylated at C4 rather than C5.
P(tBu)2Me•HBF4 was used in place of PCy2Ph.
