TABLE 3.
Synthesis of Other Heterocycles Using Aryl Chlorides as Electrophilesa
| entry | substrate | product | yieldb |
|---|---|---|---|
| 1 |
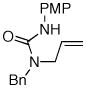 8 |
 14 |
77% |
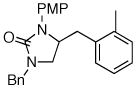
| 2 |
 9 |
 15 |
81% (20:1 dr) |
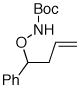
| 3 |
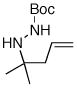 10 |
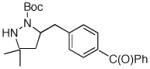 16 |
73% |
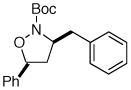
| 4 |
11 |
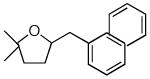 17 |
89%c |
| 5 |
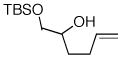 12 |
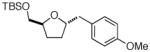 Not observed 18 |
0% |
| 6 |
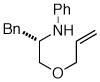 13 |
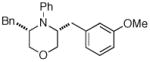 19 Not observed |
0% |
Conditions: substrate (1 equiv), aryl chloride (1.2 equiv), NaOtBu (1.2 equiv), Pd(OAc)2 (2 mol %), S-Phos (4 mol %), toluene (0.25 M), 90 °C or 110 °C.
Isolated yield (average of two or more experiments).
This material was obtained as a 13:1 mixture of regioisomers.
