Abstract
Ovulation has long been recognized as one of the most dramatic reproductive processes. Decades of research on how the LH/FSH surge leads to ovulation have made it clear that the surge induces a very complex cascade of changes. Studies of genetically modified mice have pointed to progesterone (P4) and its receptor (PGR) and the prostaglandins (PGs) as critical components of the ovulatory cascade. In cattle, the gonadotropin surge also induces oxytocin (OT), which does not appear to increase in rodent periovulatory follicles. This review is an attempt to summarize studies by our laboratory on the temporal patterns, roles, regulation, and interrelationships among P4/PGR, PGs, and OT in bovine periovulatory follicles. Most of these results are based on an experimental model in which the dominant follicle of the first follicular wave of the estrous cycle is induced to develop into a preovulatory follicle by injection of PGF2α on Day 6 of the cycle, followed 36 h later by an injection of GnRH to induce the LH/FSH surge. The results suggest that the effects of the gonadotropin surge on PG production by bovine granulosa cells are mediated by the gonadotropin-induced increase in intrafollicular P4 and that P4 acts by binding to its nuclear receptor and increasing the abundance of mRNA for the enzyme PTGS2 (COX-2). Our data thus far also support the hypothesis that PGs, especially PGE2, can stimulate progesterone secretion by both follicular cell types and suggest a positive feedback relationship between P4/PGR and the PGs. Additional results suggest a positive feedback loop between P4/PGR and OT. The finding that levels of mRNA for several ADAMTS proteases are regulated by the LH/FSH surge in vivo and by P4/PGR and/or PGs in vitro suggests a role for this family of proteases in remodeling the bovine ovulatory follicle in preparation for ovulation and the formation of the corpus luteum. It is important to remember that a process essential for reproduction, such as ovulation, may involve redundant mechanisms and that these mechanisms may have evolved differently from rodents in larger mammalian species, such as ruminants and humans.
Keywords: ovary, ovarian follicle, ovulation, cattle, progesterone, prostaglandins, ADAMTS proteases, oxytocin
Introduction
Ovulation is one of the most dramatic processes in female reproduction. Like parturition, it is a cataclysmic event that disrupts homeostasis and involves changes that would be deleterious under other circumstances (breakdown of tissue, bleeding, etc). Ovulation resembles parturition because positive feedback mechanisms that keep the follicle proceeding on the path towards rupture appear to be important, just as positive feedback loops drive the increasing strength and frequency of uterine contractions during parturition. Three major changes occur during the periovulatory period, the time between the LH/FSH surge and ovulation: meiotic maturation of the oocyte, follicular rupture and ovulation, and the shift in follicular steroidogenesis from androgen/estradiol to progesterone (P4) as the primary steroid product (i.e., the follicular luteal phase shift in steroidogenesis).
During the last decade or so, our laboratory has been interested in understanding the temporal patterns, regulation, and role of three types of hormones that change dramatically during the periovulatory period - progesterone (and its receptor, PGR), prostaglandins (PGs), and oxytocin. More recent studies have been focused on temporal changes in members of a recently discovered family of proteases, the ADAMTS (A Disintegrin And Metalloproteinase with Thrombo Spondin motifs) proteases, during the bovine periovulatory period and on their regulation by progesterone/PGR and prostaglandins. This review is an attempt to summarize the research of our laboratory on changes in hormone production by bovine ovulatory follicles during the periovulatory period, mechanisms that subserve those changes, and their roles during the periovulatory interval.
Experimental model
The bovine estrous cycle usually has two or three consecutive waves of follicular development, each producing a dominant follicle that is selected from a cohort of follicles that are recruited synchronously to grow larger than 4 mm in diameter (Savio et al., 1988; Sirois and Fortune, 1988; Ginther et al., 1989). The follicle that is functionally dominant at the time of luteal regression becomes the ovulatory follicle of that cycle. The endocrine conditions during the luteal to follicular phase transition (i.e., the decline in circulating progesterone, which allows an increase in LH pulse frequency) promote the development of the dominant follicle into a preovulatory follicle capable of making enough estradiol to trigger the gonadotropin surge. However, the dominant follicle of any wave can ovulate if the corpus luteum is regressed (e.g. via an injection of prostaglandin) during its tenure of functional dominance.
Our laboratory developed and validated an experimental model (Komar et al., 2001) that facilitates study of the events of the bovine periovulatory period (others have developed similar models). In our model, the first dominant follicle of the cycle is induced to develop into a preovulatory follicle by injection of a luteolytic dose of prostaglandin F2α (PGF2α) on the evening of Day 6 or morning of Day 7 of the cycle, followed by injection of a GnRH analog 36 h later (Komar et al., 2001). In this model, the peak of the LH surge occurs 2 h after GnRH and ovulation is detected around 29 h after GnRH, on average. The ovary bearing the preovulatory follicle is removed by colpotomy 36 h after injection of PGF2α (time 0 of GnRH) to obtain “preovulatory” follicles or at various times after injection of GnRH to obtain “periovulatory” follicles. The ovulatory follicle is dissected from the ovary and the follicle wall (theca + granulosa cells) or isolated theca and granulosa cells are obtained and used for experiments in vitro, as described previously (Fortune and Hansel, 1979).
Progesterone and progesterone receptor: temporal changes during the bovine periovulatory period
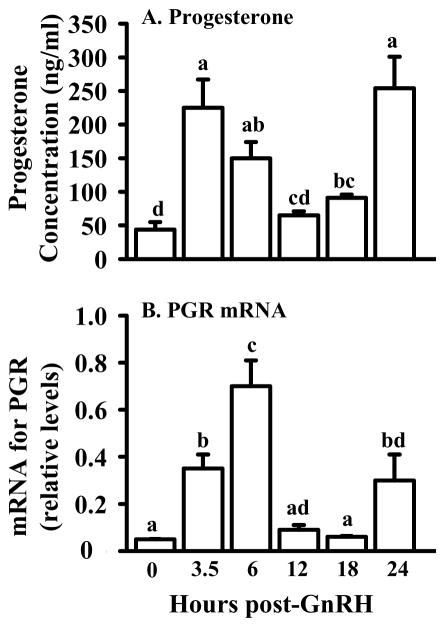
The steroidogenic capabilities of mammalian ovulatory follicles change dramatically during the periovulatory period. As shown in Fig. 1A, concentrations of progesterone in the follicular fluid of bovine ovulatory follicles increase about 4.5-fold between time 0 and 3.5 h after GnRH (1.5 h after the peak of the LH surge). By 12 h post-GnRH, levels return to those observed at time 0 and then a second increase in progesterone is evident at 24 h, close to the time of ovulation (Fig. 1A; Fortune et al., 2001). In contrast, concentrations of androstenedione, testosterone, and estradiol are unchanged at 3.5 h post-GnRH, compared with 0 h, and exhibit gradual but dramatic decreases thereafter (Komar et al., 2001). The changes in the concentrations of P4 are reflected in a fairly similar pattern of change in levels of mRNA for the progesterone receptor (PGR) in follicular wall tissue (theca + attached granulosa cells), with a peak in abundance of mRNA at 6 h and a later increase at 24 h after injection of GnRH (Fig. 1B; Jo et al., 2002). In experiments in vitro, both isolated theca and granulosa cells exhibited transient increases in mRNA for PGR in response to luteinizing doses (100 ng/ml) of LH (theca cells) or either LH or FSH (granulosa cells; Jo et al., 2002). In contrast, Cassar et al. (2002) observed an increase in mRNA at 6 h post GnRH, but not at 24 h and they localized the increase at 6 h to the granulosa cells, but not the theca, by in situ hybridization. These discrepancies may be due to the sensitivity of the methods used to detect mRNA (their northern blot/dot blot vs. our northern blot/RNase protection assay). At least the early increase in PGR mRNA is transient and therefore, small differences in timing of short-lived periovulatory changes due to the use of slightly different animal models may be a factor. The increase in mRNA that Jo et al. (2002) detected in theca cells in vitro was too consistent and robust to be easily attributed to contamination of the thecal preparation with granulosa cells.
Figure 1.
Concentrations of progesterone (ng/ml ± SEM) in follicular fluid (A) and abundance of mRNA (B) for progesterone receptor (PGR) in follicle wall tissue of bovine pre/periovulatory follicles obtained at 0, 3.5, 6, 12, 18, or 24 h after injection of GnRH to induce an LH/FSH surge. Levels of PGR mRNA were determined by RNase protection assay and relative levels were calculated by correcting for the intensity of the 18S rRNA band in each sample. Values are means ± SEM (n = 3 follicles/time point). Within each panel values with no common superscripts differ (P < 0.05). (Data are derived from Fortune et al., 2001 and Jo et al., 2002).
Although the increase in follicular progesterone production and concomitant decreases in androgens and estradiol discussed above reflect a shift from a follicular to a luteal steroidogenic profile, results of a number of studies have suggested that P4 plays an essential role in ovulation. Progesterone has been suggested for decades as a mediator of the ovulatory response. Anti-progesterone antiserum blocks ovulation in rats and the block can be overcome by progesterone, but not estradiol (Mori et al., 1977). Likewise, inhibitors of enzymes necessary for progesterone synthesis, P450 side chain cleavage (P450scc) and 3β-hydroxysteroid dehydrogenase (3β-HSD), inhibit ovulation and the block can be overcome with exogenous progesterone (Lipner and Greep, 1971; Snyder et al., 1984). However, an obligatory role for progesterone in ovulation was controversial, since other studies did not support such a role (reviewed in Espey and Lipner, 1994). The production and characterization of mice null mutant for progesterone receptor (PGR) provided new support for an essential role for progesterone (Lydon et al., 1995). In PGR-deficient mice, ovulatory-size follicles develop but fail to ovulate, even in response to an ovulatory dose of gonadotropins (Lydon et al., 1995). In cattle injection of an inhibitor of progesterone synthesis (trilostane) into the preovulatory follicle just after injection of GnRH inhibited the increase in P4 in the follicular fluid at 24 h post-GnRH, but did not block follicular rupture (Li et al., 2007). It is not known if the early rise in P4 in the follicular fluid was also inhibited. The coordinate changes during the bovine periovulatory period in P4 concentrations in bovine follicular fluid and levels of PGR mRNA in follicular tissue (Fig. 1) suggest that progesterone, acting through its nuclear receptor, may have both early and later effects on the ovulatory follicle. Some of the potential effects of P4/PGR on periovulatory bovine follicles are discussed in succeeding sections.
Prostaglandins: temporal changes during the bovine periovulatory period
Evidence that prostaglandins are key regulators of the ovulatory process has been accumulating for almost 30 years. Inhibitors of PG synthesis, such as indomethacin, block ovulation in a variety of species, including rats, rabbits, mice, sheep, pigs, cattle, and primates (reviewed in Armstrong, 1981; Murdoch et al., 1993) and indomethacin also blocks ovulation in isolated, perfused rat and rabbit ovaries (Hamada et al., 1977; Brännström et al., 1987). In addition, PGE2 and PGF2α increase dramatically in follicular fluid close to the time of follicular rupture (reviewed in Armstrong, 1981; Murdoch et al., 1993; Priddy and Killick, 1993) and specific antisera to PGs block ovulation in rabbits and mice (Armstrong et al., 1974; Lau et al., 1974). Doubt was cast on an obligatory role for PGs in ovulation by the failure of PG administration in some studies to overcome the ovulatory blockade of indomethacin (reviewed in Murdoch et al., 1993; Espey and Lipner, 1994). However, more recently two lines of experimental evidence have provided new and compelling support for a critical role for PGs in ovulation. First, the induction by the gonadotropin surge of a distinct and inducible form of prostaglandin G/H synthase, PTGS2 (also known as cyclooxygenase-2 or COX-2), which converts arachidonic acid to PGH2, was described in follicles of several species, including rats, cattle, and horses (Sirois et al., 1992; Sirois, 1994; Sirois and Doré, 1997). Second, mice deficient in PTGS2 (COX-2 knockout mice) are infertile; ovulation is severely impaired and administration of gonadotropins does not restore ovulatory capacity (Dinchuk et al., 1995; Lim et al., 1997). In contrast, mice lacking the constitutive form of the enzyme (PTGS1 or COX-1) are fertile and appear to ovulate normally (Langenbach et al., 1995). Thus, an increase in intrafollicular production of PGs in response to the induction of PTGS2 appears essential for the ovulatory process, but the exact role of the PGs in promoting ovulation is still unclear.
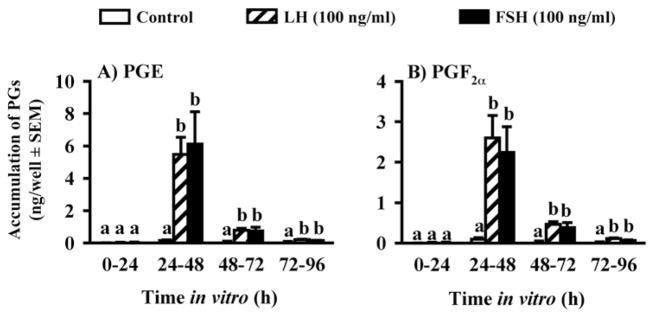
We used the experimental model described above to first determine the time course of increases in PGF2α and PGE in periovulatory bovine follicles generated using the model. The concentrations of both PGs were very low in follicular fluid until 24 h post-GnRH (Fig. 2; Bridges et al., 2006), which suggests that PG secretion does not commence until late in the periovulatory period, between 18 and 24 h after GnRH. These data are consistent with an earlier report for cattle by Sirois (Sirois, 1994). When follicle wall tissue, obtained at 0, 3.5, 6, 12, or 24 h, was cultured for 8 h, there was a significant, but transient increase in PG secretion in vitro by follicle wall tissue obtained at 3.5 h post-GnRH, but not by tissue isolated at 0, 6, or 12 h, and a sustained increase by tissue isolated 18 h post-GnRH that was more pronounced in follicle wall obtained at 24 h (Bridges et al., 2006). In longer-term cultures of follicle wall, PG secretion was always transient and the time of peak secretion was inversely related to the time when the tissue was obtained relative to the injection of GnRH. Interestingly, the total accumulation of PG over 3 days of culture was greater as tissue was isolated later in the periovulatory period, suggesting that there are events in vivo that are not replicated in vitro. When theca and granulosa cells were isolated and cultured separately in the absence or presence of gonadotropins, there was significant secretion of the PGs only in cultures of granulosa cells. Both LH and FSH increased the secretion of the PGs by granulosa cells very dramatically, with peak effects on the second day of culture (Fig. 3; Bridges et al., 2006). Previous studies have shown that the increase in PGs in the follicular fluid of bovine periovulatory follicles is associated with induction of mRNA and protein for PTGS2 (COX-2) in granulosa, but not theca, cells of ovulatory follicles (Sirois, 1994).
Figure 2.
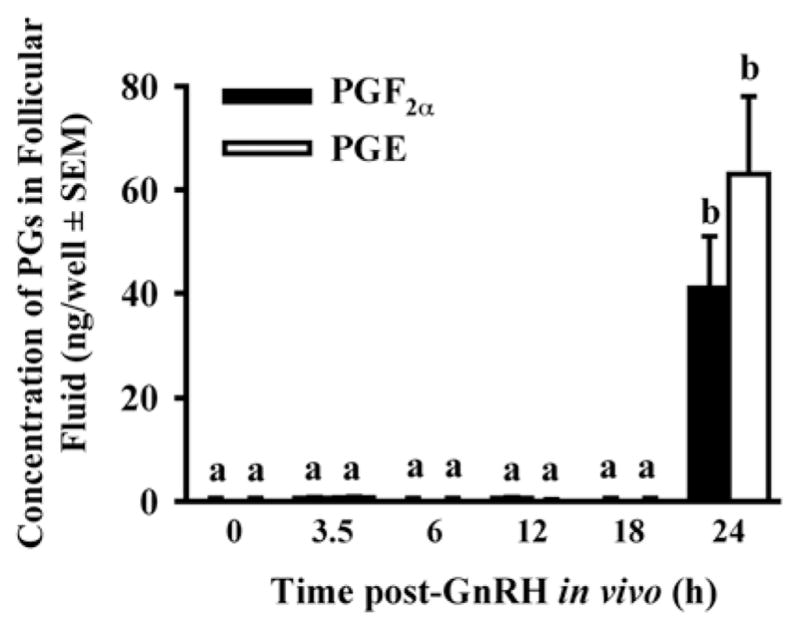
Concentrations of PGE and PGF2α in the follicular fluid of preovulatory follicles collected at 36 h after PGF2α (0 h post-GnRH) and periovulatory follicles collected at 3.5, 6, 12, 18 and 24 h after heifers were injected with GnRH to induce the LH/FSH surge. Data are means ±SEM of 3 follicles per time point. For each PG, values with different superscripts differ (P < 0.05). (Bridges et al., 2006, with permission).
Figure 3.
Secretion of PGE (A) and PGF2α (B) by granulosa cells from preovulatory follicles collected at 36 h after PGF2α (0 h post-GnRH) and cultured for 96 h with 0 or 100 ng/ml LH or FSH. Data are the means ± SEM of duplicate cultures from each of 4 preovulatory follicles. In each panel, within a time in vitro values with different superscripts differ (P < 0.05). (Data are derived from Bridges et al., 2006).
In addition to inducing prostaglandin synthesis, the LH/FSH surge also modulates the levels of mRNA for receptors for PGF2α (FP) and for PGE2 (EP2, EP3, and EP4). The temporal patterns of these changes are quite complicated since they vary by both receptor type and cell type (Bridges and Fortune, 2007). These results suggest that both theca and granulosa cells of periovulatory follicles are targets for both the PGF2α and the PGE2 produced by the granulosa cells, if the changes in abundance of mRNA are matched by corresponding increases/decreases in receptor protein, but the functional meaning of these complicated and rapid changes has yet to be elucidated. In addition, our studies with periovulatory follicles have provided evidence for changes in the levels of mRNA for bovine prostaglandin transporter (bPGT). Although it had been assumed that PGs are transported through the cell membrane and within the intercellular space by passive diffusion, recent studies have provided evidence for a prostaglandin transporter in rats (Kanai et al., 1995), humans (Lu et al., 1996), and cattle (Banu et al., 2003). Our results showed that mRNA for bPGT is upregulated about fivefold by the LH/FSH surge in granulosa cells of periovulatory follicles at all times examined (6, 12, 18, and 24 h post-GnRH). The bPGT mRNA was considerably more abundant in theca cells than in granulosa cells and in the theca it was slightly, but significantly, lower at 18 and 24 h post-GnRH than at earlier time points (Bridges and Fortune, 2007). These results suggest that changes in the level of bPGT available to transport PGs may also modulate the actions of PGs on the periovulatory follicle. For example, production of bPGT by granulosa cells may increase the efflux of PGs from the cells and thus, their availability for autocrine and paracrine actions within the follicle; production of bPGT by theca cells may serve to increase their access to the PGs made by the granulosa cells.
Although experimental evidence suggests that the increase in PGs in bovine periovulatory follicles is essential for ovulation (Peters et al., 2004), the actions of PGs on bovine periovulatory follicles are still being studied. There is some evidence for rats and sheep that they mediate the gonadotropin-dependent increase in interstitial collagenase that occurs prior to ovulation (Murdoch et al., 1986; Reich et al., 1991) and they may also promote a highly localized inflammatory response in the follicle prior to ovulation (Priddy and Killick, 1993). Experiments by Dr. G.W. Smith and his colleagues have linked the increase in follicular PGs with specific effects on proteolytic enzymes in periovulatory follicles and on their inhibitors (Li et al., 2006).
Regulation of prostaglandins and progesterone during the periovulatory period
Experimental evidence shows that the up-regulation of progesterone/PGR and the induction of PG production are a result of the gonadotropin surge. These increases occur at specific times in vivo after the surge and the transient induction of PG production by granulosa cells (Bridges et al., 2006) and at least the first transient increase in mRNA for PGR in theca and granulosa cells (Jo et al., 2002) can be induced in vitro with luteinizing doses (100 ng/ml) of the appropriate gonadotropin(s). Once the timing of the increases in the PGs and P4/PGR mRNA were determined, we began to test hypotheses about how these effects of the LH/FSH surge are mediated.
Effects of P4/PGR on follicular production of prostaglandins
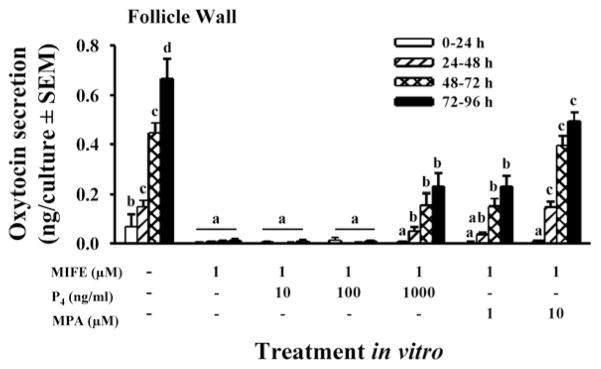
First we tested the hypothesis that the increase in P4/PGR is essential for the induction of PG secretion by granulosa cells (Bridges et al., 2006). Granulosa cells were isolated from preovulatory follicles 36 h after PGF2α injection (time 0 of GnRH) and cultured with a luteinizing dose of LH (to induce PGR) in the presence or absence of the PGR antagonist mifepristone (RU-486). Mifepristone completely inhibited LH-induced production of PGE and PGF2α (Fig. 4). Although mifepristone is reported to also bind to the glucocorticoid receptor, its actions in inhibiting PG production by bovine granulosa cells appear to be specific since the synthetic progestin MPA completely overcame mifepristone’s effects, whereas the synthetic glucocorticoid dexamethasone did not (Fig. 4). In addition, mifepristone also inhibited the LH-induced increase in mRNA for PTGS2 (COX-2) and MPA overcame that inhibitory effect (Fig. 5). Taken together these finding strongly suggest that the effects of the gonadotropin surge on PG production by bovine granulosa cells are mediated by the gonadotropin-induced increase in intrafollicular progesterone and that P4 acts by binding to its nuclear receptor and increasing the abundance of mRNA for PTGS2. We hypothesize that the early increase in intrafollicular progesterone (Fig. 1) may be responsible for the increase in PG production by granulosa cells between 18 and 24 h post-GnRH. The report by Li et al. (2007) that injection of an inhibitor of progesterone synthesis into the preovulatory follicle blocked the effects of the gonadotropin surge on both progesterone and the periovulatory increase in PGs in the follicular fluid is consistent with that hypothesis.
Figure 4.
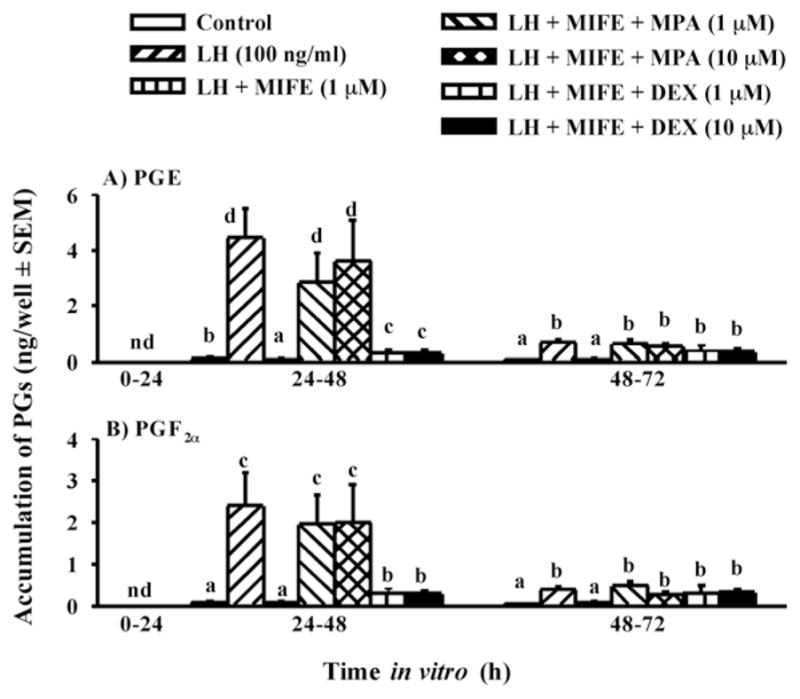
Secretion of PGE (A) and PGF2α (B) by granulosa cells from preovulatory follicles collected at 0 h post-GnRH and cultured for 96 h in medium alone or with LH (100 ng/ml), LH + mifepristone (MIFE, 1 μM), LH + MIFE + medroxyprogesterone acetate (MPA, 1 or 10 μM), and LH + MIFE + dexamethasone (DEX, 1 or 10 μM). Panels A and B show accumulation of PGs during the first 72 h of culture; treatment effects from 72–96 h were similar to 48–72 h, but absolute values were very low (data not shown). Data are the means ± SEM of duplicate cultures from each of 5 preovulatory follicles. nd = not detectable. In each panel, within a time in vitro values with no common superscript differ (P < 0.05). (Data are derived from Bridges et al., 2006).
Figure 5.
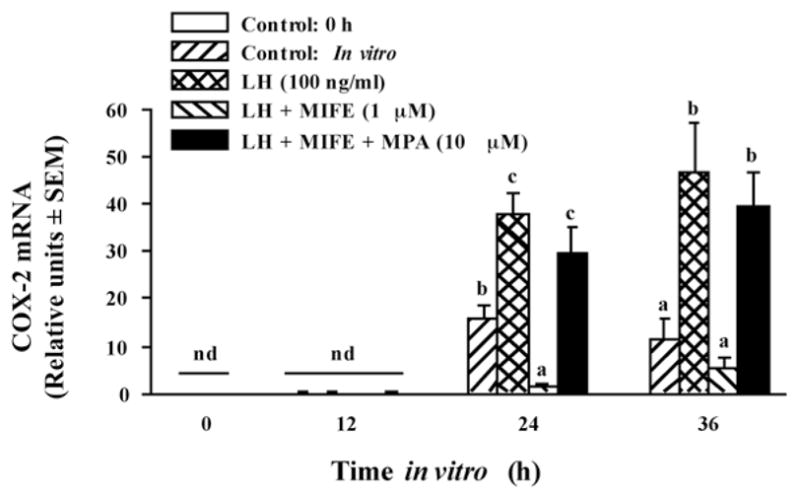
Inhibition of LH-induced mRNA for COX-2 (also known as PTGS2) by mifepristone (MIFE) is overcome by concurrent progestogen (MPA) treatment. Relative levels of mRNA for COX-2 were determined by semi-quantitative RT-PCR and calculated by correcting for the intensity of the band for the 18S subunit of rRNA in each sample. Data are the means ± SEM of cultures from each of 4 preovulatory follicles. nd = not detectable. Within a time in vitro values with different superscripts differ (P < 0.05). (Data are derived from Bridges et al., 2006).
Effects of PGs on progesterone production by follicular cells
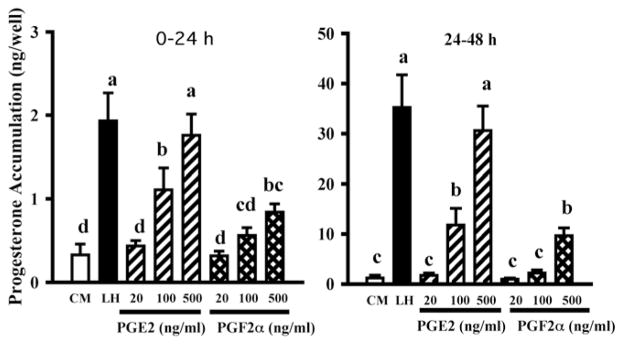
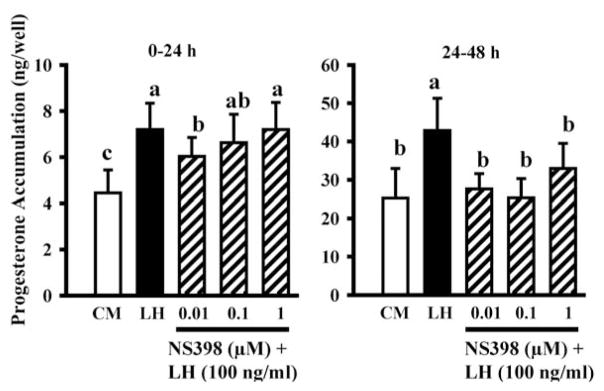
Progesterone/PGR and PGs both increase late in the periovulatory period (Figs. 1 and 2) and this raised the possibility that PGs might play a role in the second periovulatory increase in progesterone. Therefore, we tested the hypothesis that PGE2 stimulates P4 secretion by culturing periovulatory theca and granulosa cells (isolated 24 h after GnRH) with PGE2 (100 ng/ml). Progesterone production by both cell types increased with time in culture, as expected, and PGE2 enhanced P4 production by theca cells by about four- to sevenfold, but had no effect on granulosa cells (Bridges and Fortune, 2007). To follow up on this interesting finding, we cultured theca interna obtained at 0 h or 24 h post-GnRH with graded doses of PGE2 and PGF2α (Fortune et al., 2008). Both PGs stimulated progesterone secretion in a dose-dependent manner, but the theca was more sensitive and more responsive to PGE2 (Fig. 6 shows results for tissue obtained at 0 h). We hypothesized that the granulosa cells may not have responded to PGs when they were isolated at 24 h after injection of GnRH in the earlier study (Bridges and Fortune, 2007) because they are making PGs at that time. To test that hypothesis, we cultured granulosa cells obtained at time 0 of GnRH with LH in the presence or absence of graded doses of a specific PTGS2 inhibitor, NS398. On the second day of culture, when the production of PGs by the granulosa cells is at its peak (see Fig. 3), NS398 completely inhibited the effects of LH on progesterone accumulation in the cultures (Fig. 7). Therefore, our data thus far support the hypothesis that PGs, especially PGE2, can stimulate progesterone secretion by both follicular cell types and suggest a positive feedback relationship between P4/PGR and PGs (see Fig. 11). However, in apparent conflict with that suggestion, Peters et al. (2004) showed that intrafollicular injection of NS398 blocked follicular rupture and greatly reduced the concentration of PGE in follicular fluid at 24 h post-GnRH, without reducing the level of P4 in the follicular fluid at 24 h post-GnRH.
Figure 6.
Accumulation of progesterone in cultures of theca interna cells isolated from preovulatory follicles 36 h after injection of PGF2α to induce luteal regression and cultured in control medium (CM), with a luteinizing dose of LH (100 ng/ml), or with graded doses of PGE2 or PGF2α. Data are means ± SEM of duplicate cultures from each of 5 follicles. Within each time in vitro, values with different superscripts differ (P < 0.05).
Figure 7.
Accumulation of progesterone in cultures of granulosa cells isolated from preovulatory follicles 36 h after injection of PGF2α to induce luteal regression and cultured in control medium (CM), with a luteinizing dose of LH (100 ng/ml), or LH + graded doses of NS398 (specific PTGS2 inhibitor). Data are means ± SEM of duplicate cultures from each of 5 follicles. Within each time in vitro, values with different superscripts differ (P < 0.05).
Figure 11.
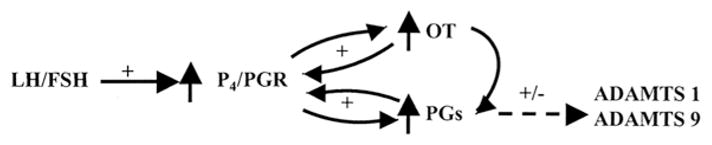
Hypothesized model of the relationships among progesterone/progesterone receptor (P4/PGR), prostaglandins (PGs), oxytocin (OT), and ADAMTS proteases in bovine periovulatory follicles, based on data discussed in the manuscript. Positive effects and potential intra-follicular positive feedback loops are indicated by “+”, whereas “+/−” indicates that the effect varies depending on the putative regulator, ADAMTS subtype, and follicular cell type.
Interactions among P4/PGR, prostaglandins, and oxytocin
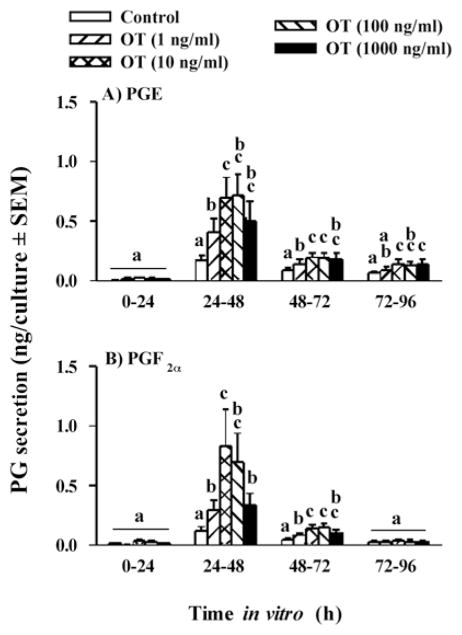
The LH/FSH surge in vivo and luteinizing doses of gonadotropins in vitro also induce the secretion of the nonapeptide hormone oxytocin (OT) by granulosa cells of the periovulatory follicle via an increase in OT mRNA (Voss and Fortune, 1991a; Voss and Fortune, 1992). Lioutas et al. (1997) reported that antagonists to the PGR inhibited the upregulation of OT mRNA and OT in granulosa cells from preovulatory-size follicles obtained from an abattoir. We showed the requirement for P4/PGR is also present in vivo in granulosa cells that have been exposed to the gonadotropin surge (Bridges and Fortune, 2007). When granulosa cells were isolated 6 h post-GnRH, mifepristone inhibited their secretion of OT and both MPA and natural progesterone overcame that inhibition in a dose-dependent manner, with similar dose-response relationships (Fig. 8). When granulosa cells were exposed to graded doses of OT in vitro, OT dose-dependently increased the secretion of both PGE and PGF2α (Fig. 9). Quantitatively, OT was less effective than the gonadotropins at increasing PGs (Fig. 3 vs. Fig. 9), but it is possible that the gonadotropin surge produces changes in the granulosa cells (receptors, etc) that make them more responsive to OT by the time when endogenous OT is secreted, late in the periovulatory period (Voss and Fortune, 1991a). Our laboratory has also shown that OT increases the secretion of P4 by granulosa cells isolated from preovulatory follicles before, but not after, the gonadotropin surge (Chandrasekher and Fortune, 1990; Voss and Fortune, 1991b). The failure of OT to stimulate progesterone production by granulosa cells obtained from follicles close to the time of ovulation may be due to the fact that endogenous production of OT by granulosa cells is ongoing at that time in vivo, so they may already be maximally stimulated. Taken together, these results suggest a positive feedback loop between P4/PGR and OT (see Fig. 11).
Figure 8.
Secretion of oxytocin in cultures of follicle wall (theca + attached granulosa cells) from periovulatory follicles collected at 6 h post-GnRH and cultured for 96 h in medium alone, or with the PGR antagonist mifepristone (MIFE, 1 μM), MIFE + progesterone (P4, 10, 100, 1000 ng/ml) or MIFE + medroxyprogesterone acetate (MPA, 1 or 10 μM). Data are the means ± SEM of duplicate cultures from each of 4 periovulatory follicles. Within a time in vitro, values with no common superscripts differ (P < 0.05; Bridges and Fortune, 2007, with permission).
Figure 9.
Secretion of PGE (A) and PGF2α (B) by granulosa cells from preovulatory follicles collected at 36 h after injection of PGF2α and cultured for 96 h with 0, 1, 10, 100 or 1000 ng/ml oxytocin. Data are the means ± SEM of duplicate cultures from each of 6 follicles. In each panel, within a time in vitro, values with different superscripts differ (P < 0.05). (Data are derived from Bridges and Fortune, 2007).
Regulation of mRNA for ADAMTS proteases by P4/PGR and PGs
It has long been recognized that proteolytic enzymes play critical roles in the process of follicular rupture and in the remodelling of the ovulatory follicle between the LH surge and ovulation (reviewed in Smith et al., 2002; Curry and Smith, 2006). Inhibitors of the proteases are also up-regulated during the periovulatory period and are believed to act to limit tissue breakdown to the apex and other appropriate parts of the ovulatory follicle, such as the basement membrane between the theca and granulosa compartments of the follicle. Plasminogen activator and its inhibitors, plasminogen, and various matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) are induced or upregulated in response to the LH/FSH surge. Dr. G.W. Smith and his collaborators have been especially active in studying this aspect of periovulatory regulation in cattle and readers are referred to their publications (Smith et al., 1996; Bakke et al., 2002, 2004; Dow et al., 2002a, b; Li et al., 2004, 2006).
More recently, evidence for a role for members of the newly described ADAMTS family of metalloproteinases during the periovulatory interval has emerged. When levels of mRNA for eight ADAMTS subtypes (1, 2, 3, 4, 5, 7, 8, and 9) were measured in theca and granulosa cells from bovine preovulatory (0 h) and periovulatory follicles (24 h post-GnRH) by semi-quantitative PCR, all, except ADAMTS4, were either up or down regulated at 24 h post-GnRH vs. time 0 in at least one follicular cell type (Madan et al., 2003). More recently, we characterized the abundance of mRNA for the ADAMTS1, 2, 7, and 9 subtypes in theca and granulosa cells isolated from pre/periovulatory follicles at 0, 6, 12, 18, and 24 h after injection of GnRH. The results showed that the abundance of mRNA for these four subtypes changes in both time- and cell-specific patterns (summarized schematically in Fig. 10). These patterns suggest that the roles of ADAMTS proteases in bovine periovulatory follicles are complex and potentially myriad. To begin to study how the abundance of mRNA for ADAMTS proteases is regulated by the LH surge, we focused on ADAMTS1 and 9 because the steady-state levels of mRNA for these two ADAMTS subtypes are upregulated after the LH/FSH surge in both follicular cell types. When theca and granulosa cells were isolated 36 h after initiation of luteolysis (time 0 of GnRH) and cultured with luteinizing doses (100 ng/ml) of gonadotropins, mRNAs for these two subtypes were regulated in vitro in patterns that reflected the patterns of change observed in vivo. Theca cells responded to LH, whereas granulosa cells responded to either LH or FSH, with the two gonadotropins producing similar effects.
Figure 10.
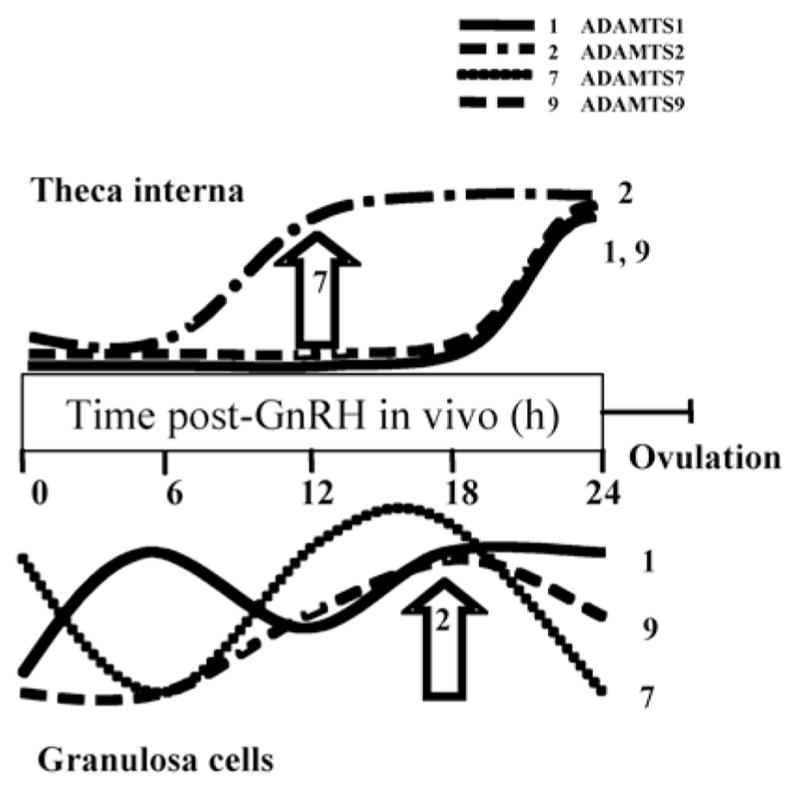
Schematic representation of changes in abundance of mRNA for ADAMTS subtypes in theca interna and granulosa cells isolated from bovine pre/periovulatory follicles between the injection of GnRH and ovulation. The large arrows indicate a transient change in ADAMTS7 and 2 at one time point.
Next, we used the in vitro model described above to test the hypothesis that P4/PGR and/or the PGs (PGE2 and/or PGF2α) are involved in mediating the effects of the LH/FSH surge on mRNA for ADAMTS1 and 9. To determine if progesterone and its receptor play a role, theca and granulosa cells obtained from preovulatory follicles at time 0 of GnRH were treated with control medium, a luteinizing dose of LH (to induce PGR), LH + the PGR inhibitor mifepristone (RU-486), or LH + mifepristone + MPA (a synthetic progestin). Mifepristone blocked the effects of LH on abundance of mRNA for ADAMTS1 and 9 mRNA in thecal cultures and on ADAMTS9 in cultures of granulosa cells, but it produced effects opposite to those of LH on ADAMTS 1 in cultures of granulosa cells (summarized in Table 1). In all cases, inclusion of MPA in the culture medium reversed the action of mifepristone and produced results similar to those of LH alone. The potential effects of PGs on mRNAs for ADAMTS1 and 9 were tested in thecal cultures by comparing cultures treated with control medium, a luteinizing dose of LH, PGE2, or PGF2α. Both PGs mimicked the effects of LH by increasing levels of mRNA for ADAMTS1, but only PGE2 mimicked the effect of LH by increasing mRNA for ADAMTS9 (Table 1). Since production of PGE and PGF2α is induced in granulosa cells by the gonadotropin surge, the potential effects of PGs on mRNA for ADAMTS1 and 9 were tested by culturing granulosa cells from preovulatory follicles with LH + graded doses of the specific COX-2 inhibitor NS398. All three doses of the inhibitor decreased both PGs to the levels observed in control medium (data not shown) and inhibited the LH-induced increase in mRNA for ADAMTS1 during the second day of culture (the time when PGs are transiently secreted by granulosa cells in vitro), but had no effect on mRNA for ADAMTS9 (Table 1).
Table 1.
Summary of results of experiments to determine if P4/PGR and/or PGs mediate the effects of the LH/FSH surge on levels of mRNA for ADAMTS1 and 91.
Granulosa and theca cells were isolated from preovulatory follicles 36 h after a luteolytic dose of PGF2α and cultured for 12, 24, 36, and 48 h. “+”, results indicate that the hormone (P4 or PG) has a positive effect on levels of the mRNA, mimicking the effects of gonadotropins; “−”, results indicate an effect that did not mimic that of the gonadotropin; NC, results show no effect.
Granulosa or theca cells were cultured with LH (100 ng/ml), LH + mifepristone (PGR inhibitor), or LH + mifepristone + MPA (synthetic progestin).
Granulosa cells were cultured with LH (100 ng/ml) + graded doses of the COX-2 inhibitor NS398 (0.01, 0.1, and 1 μM).
Theca cells were cultured with PGE2 or PGF2α (1 μM).
Our results suggest that P4/PGR and PGs may regulate mRNA for ADAMTS proteases and that their effects may be negative or positive and are time-, ADAMTS subtype- and cell type-specific. ADAMTS proteases degrade a variety of substrates, which vary depending on the subtype. ADAMTS1 and 9 share an identical enzymatic domain and both have been shown to degrade proteoglycans (e.g., versican; Kuno et al., 2000; Rodriguez-Manzaneque et al., 2002; Somerville et al., 2003). Since versican is a component of the basement membrane in bovine follicles (McArthur et al., 2000), it is possible that these proteases are involved in dissolution of the basement membrane to allow intermixing of the theca and granulosa cells as the corpus luteum forms. More research is needed to expand these initial studies on the regulation of ADAMTS subtypes in bovine periovulatory follicles and research on their specific effects within bovine follicles is completely lacking thus far.
Summary
Rodent species, especially mice, provide powerful genetic models and analysis of those models has suggested obligatory roles for the periovulatory increases in P4/PGR and PGs. However, the larger size of the ovulatory follicles of species of practical interest, such as cattle and humans, and the longer periovulatory interval may have led to variations on the “themes” that have emerged from research with rodents. Thus, studies of the intrafollicular changes that precede ovulation in these species seems essential. The results of our studies on P4/PGR, the prostaglandins, and OT during the periovulatory period have suggested positive feedback relationships among these hormones that are summarized in Fig. 11. Since ovulation and luteinization are processes in which homeostasis is not maintained, it is logical that positive feedback mechanisms would be present. Studies on ADAMTS proteases have suggested that both P4/PGR and the PGs play a role in the complex periovulatory changes in those proteases. It is logical that an event like ovulation, which is essential for successful reproduction, may involve redundant mechanisms and it is possible that this may be the reason for at least some of the apparent contradictions among studies. It seems clear that the cascade of changes that lead to bovine ovulation and luteinization is complex and far from completely understood.
Acknowledgments
Research by the authors’ lab was supported by the National Institutes of Health (HD41592 and HD14584), the U.S. Dept. of Agriculture, and a fellowship from the Lalor Foundation (to PJB). The contributions of Drs. M. Jo and C.M. Komar to the previously published results presented here are gratefully acknowledged. We thank Drs. P.A. Johnson and G.W. Smith for reading the manuscript and helpful comments and Dr. M. Yang for help in preparing the figures.
References
- Armstrong DT, Grinwich DL, Moon YS, Zamecnik J. Inhibition of ovulation in rabbits by intrafollicular injection of indomethacin and prostaglandin F antiserum. Life Sci. 1974;14:129–140. doi: 10.1016/0024-3205(74)90252-5. [DOI] [PubMed] [Google Scholar]
- Armstrong DT. Prostaglandins and follicular functions. J Reprod Fertil. 1981;62:283–291. doi: 10.1530/jrf.0.0620283. [DOI] [PubMed] [Google Scholar]
- Bakke LJ, Dow MP, Cassar CA, Peters MW, Pursley JR, Smith GW. Effect of the preovulatory gonadotropin surge on matrix metalloproteinase (MMP)-14, MMP-2, and tissue inhibitor of metalloproteinases-2 expression within bovine periovulatory follicular and luteal tissue. Biol Reprod. 2002;66:1627–1634. doi: 10.1095/biolreprod66.6.1627. [DOI] [PubMed] [Google Scholar]
- Bakke LJ, Li Q, Cassar CA, Dow MP, Pursley JR, Smith GW. Gonadotropin surge-induced differential upregulation of collagenase-1 (MMP-1) and collagenase-3 (MMP-13) mRNA and protein in bovine preovulatory follicles. Biol Reprod. 2004;71:605–612. doi: 10.1095/biolreprod.104.027185. [DOI] [PubMed] [Google Scholar]
- Banu SK, Arosh JA, Chapdelaine P, Fortier MA. Molecular cloning and spatio-temporal expression of the prostaglandin transporter: a basis for the action of prostaglandins in the bovine reproductive system. Proc Natl Acad Sci USA. 2003;100:11747–11752. doi: 10.1073/pnas.1833330100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännström M, Koos RD, LeMaire WJ, Janson PO. Cyclic adenosine 3′,5′-monophosphate-induced ovulation in the perfused rat ovary and its mediation by prostaglandins. Biol Reprod. 1987;37:1047–1053. doi: 10.1095/biolreprod37.5.1047. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Komar CM, Fortune JE. Gonadotropin-induced expression of messenger ribonucleic acid for cyclooxygenase-2 and production of prostaglandins E and F2α in bovine preovulatory follicles are regulated by the progesterone receptor. Endocrinology. 2006;147:4713–4722. doi: 10.1210/en.2005-1575. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Fortune JE. Regulation, action and transport of prostaglandins during the periovulatory period in cattle. Mol Cell Endocrinol. 2007;263:1–9. doi: 10.1016/j.mce.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Cassar CA, Dow MP, Pursley JR, Smith GW. Effect of the preovulatory LH surge on bovine follicular progesterone receptor mRNA expression. Domest Anim Endocrinol. 2002;22:179–187. doi: 10.1016/s0739-7240(02)00124-8. [DOI] [PubMed] [Google Scholar]
- Chandrasekher YA, Fortune JE. Effects of oxytocin on steroidogenesis by bovine theca and granulosa cells. Endocrinology. 1990;127:926–933. doi: 10.1210/endo-127-2-926. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24:228–241. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- Dow MP, Bakke LJ, Cassar CA, Peters MW, Pursley JR, Smith GW. Gonadotrophin surge-induced upregulation of mRNA for plasminogen activator inhibitors 1 and 2 within bovine periovulatory follicular and luteal tissue. Reproduction. 2002a;123:711–709. [PubMed] [Google Scholar]
- Dow MP, Bakke LJ, Cassar CA, Peters MW, Pursley JR, Smith GW. Gonadotropin surge-induced up-regulation of the plasminogen activators (tissue plasminogen activator and urokinase plasminogen activator) and the urokinase plasminogen activator receptor within bovine periovulatory follicular and luteal tissue. Biol Reprod. 2002b;66:1413–1421. doi: 10.1095/biolreprod66.5.1413. [DOI] [PubMed] [Google Scholar]
- Espey LL, Lipner H. Ovulation. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York, USA: Raven Press; 1994. pp. 725–780. [Google Scholar]
- Fortune JE, Hansel W. The effects of 17β-estradiol on progesterone secretion by bovine theca and granulosa cells. Endocrinology. 1979;104:1834–1838. doi: 10.1210/endo-104-6-1834. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Rivera GM, Komar CM. Selection and differentiation of dominant ovarian follicles in cattle. In: Rosa e Silva AAM, editor. BR Monographs of Reproduction & Catalog Group 2001. Sao Paulo, Brazil: Editora Arte & Ciencia/Villipress; 2001. pp. 21–38. [Google Scholar]
- Fortune JE, Yang CS, Willis ER. Prostaglandins stimulate progesterone production in vitro by theca and granulosa cells from ovulatory follicles of cattle. Proceedings of the 41st annual meeting of the Society for the Study of Reproduction; Kailua-Kona, Hawaii. 2008; Madison, WI: SSR/Biol Reprod Special Issue; 2008. pp. 180–181. [Google Scholar]
- Ginther OJ, Knopf L, Kastelic JP. Ovarian follicular dynamics in heifers during early pregnancy. Biol Reprod. 1989;41:247–254. doi: 10.1095/biolreprod41.2.247. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Bronson RA, Wright KH, Wallach EE. Ovulation in the perfused rabbit ovary: the influence of prostaglandins and prostaglandin inhibitors. Biol Reprod. 1977;17:58–63. doi: 10.1095/biolreprod17.1.58. [DOI] [PubMed] [Google Scholar]
- Jo M, Komar CM, Fortune JE. Gonadotropin surge induces two separate increases in messenger RNA for progesterone receptor in bovine preovulatory follicles. Biol Reprod. 2002;67:1981–1988. doi: 10.1095/biolreprod.102.004366. [DOI] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- Komar CM, Berndtson AK, Evans ACO, Fortune JE. Decline in circulating estradiol during the periovulatory period is correlated with decreases in estradiol and androgen, and in messenger RNA for P450 aromatase and P450 17α-hydroxylase, in bovine preovulatory follicles. Biol Reprod. 2001;64:1797–1805. doi: 10.1095/biolreprod64.6.1797. [DOI] [PubMed] [Google Scholar]
- Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000;478:241–245. doi: 10.1016/s0014-5793(00)01854-8. [DOI] [PubMed] [Google Scholar]
- Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Lau IF, Saksena SK, Chang MC. Prostaglandins F and ovulation in mice. J Reprod Fertil. 1974;40:467–469. doi: 10.1530/jrf.0.0400467. [DOI] [PubMed] [Google Scholar]
- Li Q, Bakke LJ, Pursley JR, Smith GW. Localization and temporal regulation of tissue inhibitors of metalloproteinases 3 and 4 in bovine preovulatory follicles. Reproduction. 2004;128:555–564. doi: 10.1530/rep.1.00282. [DOI] [PubMed] [Google Scholar]
- Li Q, Jimenez-Krassel F, Kobayashi Y, Ireland JJ, Smith GW. Effect of intrafollicular indomethacin injection on gonadotropin surge-induced expression of select extracellular matrix degrading enzymes and their inhibitors in bovine preovulatory follicles. Reproduction. 2006;131:533–543. doi: 10.1530/rep.1.00926. [DOI] [PubMed] [Google Scholar]
- Li Q, Jimenez-Krassel F, Bettegowda A, Ireland JJ, Smith GW. Evidence that the preovulatory rise in intrafollicular progesterone may not be required for ovulation in cattle. J Endocrinol. 2007;192:473–483. doi: 10.1677/JOE-06-0020. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Lioutas C, Einspanier A, Kascheike B, Walther N, Ivell R. An autocrine progesterone positive feedback loop mediates oxytocin upregulation in bovine granulosa cells during luteinization. Endocrinology. 1997;138:5059–5062. doi: 10.1210/endo.138.11.5650. [DOI] [PubMed] [Google Scholar]
- Lipner H, Greep RO. Inhibition of steroidogenesis at various sites in the biosynthetic pathway in relation to induced ovulation. Endocrinology. 1971;88:602–607. doi: 10.1210/endo-88-3-602. [DOI] [PubMed] [Google Scholar]
- Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA (hPGT) J Clin Invest. 1996;98:1142–1149. doi: 10.1172/JCI118897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Madan P, Bridges PJ, Komar CM, Beristain AG, Rajamahendran R, Fortune JE, MacCalman CD. Expression of messenger ribonucleic acid for ADAMTS subtypes changes in the periovulatory follicle after the gonadotropin surge and during luteal development and regression in cattle. Biol Reprod. 2003;69:1506–1514. doi: 10.1095/biolreprod.102.013714. [DOI] [PubMed] [Google Scholar]
- McArthur ME, Irving-Rodgers HF, Byers S, Rodgers RJ. Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol Reprod. 2000;63:913–924. doi: 10.1095/biolreprod63.3.913. [DOI] [PubMed] [Google Scholar]
- Mori T, Suzuki A, Nishimura T, Kambegawa A. Inhibition of ovulation in immature rats by anti-progesterone antiserum. J Endocrinol. 1977;73:185–186. doi: 10.1677/joe.0.0730185. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Peterson TA, Van Kirk EA, Vincent DL, Inskeep EK. Interactive roles of progesterone, prostaglandins, and collagenase in the ovulatory mechanism of the ewe. Biol Reprod. 1986;35:1187–1194. doi: 10.1095/biolreprod35.5.1187. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Hansen TR, McPherson LA. A review: role of eicosanoids in vertebrate ovulation. Prostaglandins. 1993;46:85–115. doi: 10.1016/0090-6980(93)90037-8. [DOI] [PubMed] [Google Scholar]
- Peters MW, Pursley JR, Smith GW. Inhibition of intrafollicular PGE2 synthesis and ovulation following ultrasound-mediated intrafollicular injection of the selective cyclooxygenase-2 inhibitor NS-398 in cattle. J Anim Sci. 2004;82:1656–1662. doi: 10.2527/2004.8261656x. [DOI] [PubMed] [Google Scholar]
- Priddy AR, Killick SR. Eicosanoids and ovulation. Prostaglandins Leukot Essent Fatty Acids. 1993;49:827–831. doi: 10.1016/0952-3278(93)90204-a. [DOI] [PubMed] [Google Scholar]
- Reich R, Daphna-Iken D, Chun SY, Popliker M, Slager R, Adelmann-Grill BC, Tsafriri A. Preovulatory changes in ovarian expression of collagenases and tissue metalloproteinase inhibitor messenger ribonucleic acid: role of eicosanoids. Endocrinology. 1991;129:1869–1875. doi: 10.1210/endo-129-4-1869. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Savio JD, Keenan L, Boland MP, Roche JF. Pattern of growth of dominant follicles during the oestrous cycle of heifers. J Reprod Fertil. 1988;83:663–671. doi: 10.1530/jrf.0.0830663. [DOI] [PubMed] [Google Scholar]
- Sirois J, Fortune JE. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol Reprod. 1988;39:308–317. doi: 10.1095/biolreprod39.2.308. [DOI] [PubMed] [Google Scholar]
- Sirois J, Simmons DL, Richards JS. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. J Biol Chem. 1992;267:11586–11592. [PubMed] [Google Scholar]
- Sirois J. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology. 1994;135:841–848. doi: 10.1210/endo.135.3.8070377. [DOI] [PubMed] [Google Scholar]
- Sirois J, Doré M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology. 1997;138:4427–4434. doi: 10.1210/endo.138.10.5462. [DOI] [PubMed] [Google Scholar]
- Smith GW, Juengel JL, McLntush EW, Youngquist RS, Garverick HA, Smith MF. Ontogenies of messenger RNA encoding tissue inhibitor of metalloproteinases 1 and 2 within bovine periovulatory follicles and luteal tissue. Domest Anim Endocrinol. 1996;13:151–160. doi: 10.1016/0739-7240(95)00065-8. [DOI] [PubMed] [Google Scholar]
- Smith MF, Ricke WA, Bakke LJ, Dow MP, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol. 2002;191:45–56. doi: 10.1016/s0303-7207(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Snyder BW, Beecham GD, Schane HP. Inhibition of ovulation in rats with epostane, an inhibitor of 3 beta-hydroxysteroid dehydrogenase. Proc Soc Exp Biol Med. 1984;176:238–242. doi: 10.3181/00379727-176-41865. [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Voss AK, Fortune JE. Oxytocin secretion by bovine granulosa cells: effects of stage of follicular development, gonadotropins, and coculture with theca interna. Endocrinology. 1991a;128:1991–1999. doi: 10.1210/endo-128-4-1991. [DOI] [PubMed] [Google Scholar]
- Voss AK, Fortune JE. Oxytocin stimulates progesterone production by bovine granulosa cells isolated before, but not after, the luteinizing hormone surge. Mol Cell Endocrinol. 1991b;78:17–24. doi: 10.1016/0303-7207(91)90181-q. [DOI] [PubMed] [Google Scholar]
- Voss AK, Fortune JE. Oxytocin/neurophysin-I messenger ribonucleic acid in bovine granulosa cells increases after the luteinizing hormone (LH) surge and is stimulated by LH in vitro. Endocrinology. 1992;131:2755–2762. doi: 10.1210/endo.131.6.1446614. [DOI] [PubMed] [Google Scholar]