Abstract
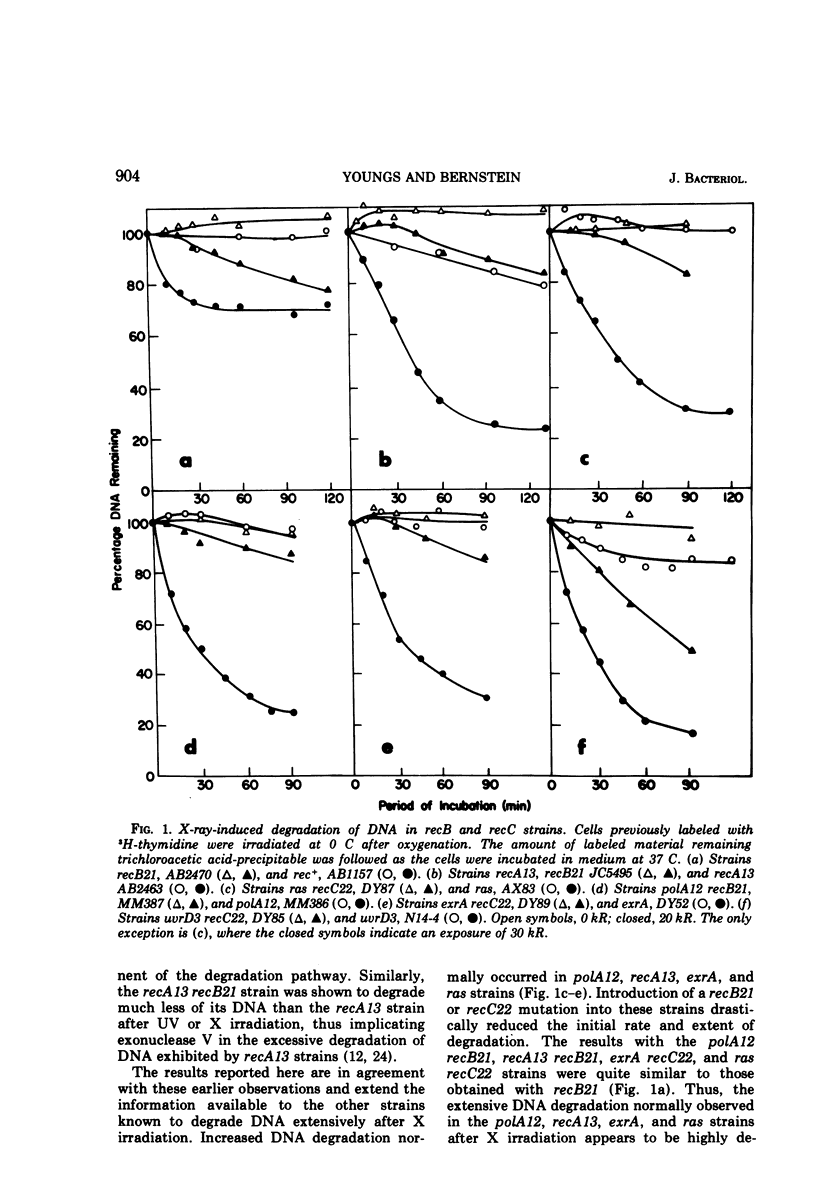
The ras, polA, exrA, recA, and uvrD3 strains of Escherichia coli K-12 degrade their deoxyribonucleic acid more extensively than wild-type strains after X irradiation. The relationship of the recB-recC nuclease (exonuclease V) to the degradation process in these strains was determined by comparing the degradation response of the original strains with that of strains containing an additional recB21 or recC22 mutation. The initial rate of degradation in ras, polA12, exrA, and recA13 strains after an exposure of 20 to 30 kR was reduced more than 10-fold by the presence of an additional recB21 or recC22 mutation. The extent of degradation in these irradiated strains after 90 to 120 min of incubation was reduced two- to fivefold. In the uvrD3 strain, a recC22 mutation caused a fourfold decrease in initial degradation rate and reduced the extent of degradation after 90 min of incubation by a factor of 1.6. The results are consistent with the statement that the degradation process is normally dependent on exonuclease V activity. However, the observation that 10 to 30% degradation always occurred even in recB or recC strains, which lack this enzyme, suggests that alternative degradation mechanisms exist.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. F. A radiation-sensitive mutant of Escherichia coli. Biochim Biophys Acta. 1958 Dec;30(3):636–637. doi: 10.1016/0006-3002(58)90112-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentzen D. E., Walker J. R. Bromodeoxyuridine sensitization of the ultraviolet-sensitive Escherichia coli ras- mutant to ultraviolet irradiation. Mol Gen Genet. 1970;108(3):218–224. doi: 10.1007/BF00283351. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W., Swartzendruber D. C. Breakdown of DNA in x-irradiated Escherichia coli. Biophys J. 1966 Jan;6(1):113–122. doi: 10.1016/S0006-3495(66)86643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Walker J. R. Escherichia coli ras locus: its involvement in radiation repair. J Bacteriol. 1969 Sep;99(3):713–719. doi: 10.1128/jb.99.3.713-719.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]


