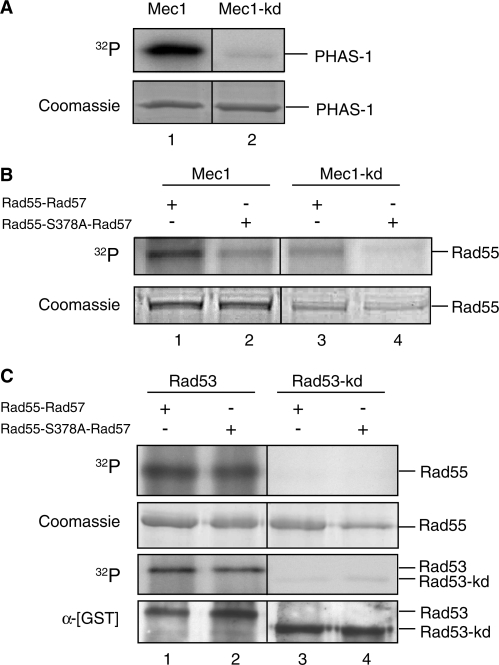
Figure 2.
Rad55 is phosphorylated in a S378-dependent manner by Mec1 but not Rad53 kinase in vitro. In vitro kinase assays. (A) The synthetic substrate PHAS-1 was incubated with immunoprecipitated HA-tagged wild-type (Mec1: lane 1) or kinase-deficient Mec1 kinase (Mec1-kd: lane 2) and analyzed using SDS–PAGE. The gel was Coomassie stained and subsequently dried. The dried gel was subjected to autoradiography. The Coomassie-stained protein bands served as a loading control. (B) Affinity chromatography purified [GST]-Rad55-[His6]-Rad57 wild-type protein (Rad55–Rad57: lanes 1, 3) or [GST]–Rad55–S378A–[His6]–Rad57 mutant protein (Rad55–S378A–Rad57: lanes 2, 4) were used as a substrate for immunoprecipitated wild-type HA-Mec1 (Mec1: lanes 1, 2) or the catalytic-deficient mutant version (Mec1-kd: lanes 3, 4). The reactions were analyzed as in (A). The same amounts of kinase and substrate were used in all reactions (Figure 3B), and the discrepancies between lanes 1/2 and 3/4 in the Coomassie panel are due to differences in staining/destaining. (C) Affinity chromatography purified wild-type GST-Rad53 kinase (Rad53: lanes 1, 2) or the catalytic-deficient mutant version (Rad53-kd: lanes 3, 4) were incubated with purified [GST]–Rad55–[His6]–Rad57 (Rad55-Rad57: lanes 1, 3) or [GST]–Rad55–S378A–[His6]–Rad57 (Rad55–S378A–Rad57: lanes 2, 4). The reaction was analyzed as in (A). Rad53 kinase activity was observed through Rad53 autophosphorylation, which became apparent after autoradiography (32P). The anti-GST immunoblot served as a loading control for [GST]–Rad53 and [GST]–Rad53–kd. Note that there is no electrophoretic shift of the GST–Rad55 fusion protein in response to phosphorylation by Rad53 kinase.