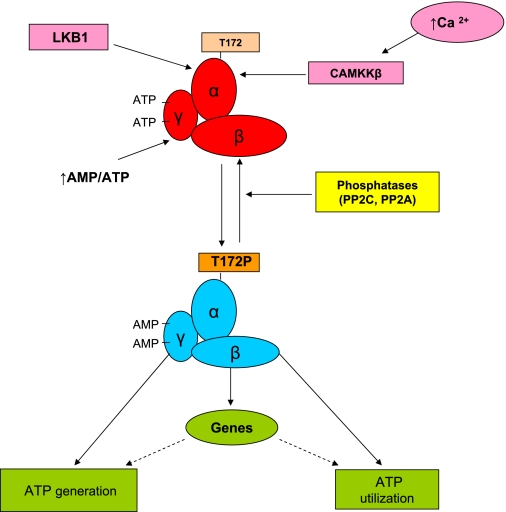
Fig. 1.
AMP-activated protein kinase (AMPK) activation and its regulation (29). AMPK is a heterotrimer consisting of a catalytic α-subunit (α1, α2) and regulatory β- and λ-subunits (β1, β2, α1, α2, and α3), all of which are required for its activity. Heterotrimers containing the α1 subunit are exclusively cytoplasmic; however, α2-containing AMPK is also found in the nucleus, where its presence may increase after exercise (56). The γ-subunit contains several cystathione β-synthase domains that under baseline conditions predominantly bind ATP. When a cell is energy stressed and the AMP/ATP ratio increases, AMP replaces ATP on 2 of these domains (83). This results in a conformational change that 1) causes a modest increase in AMPK activity (2- to 10-fold) and 2) enhances the phosphorylation of Thr172 on the α-subunit, which results in a much greater activation of the enzyme. The active enzyme then phosphorylates multiple molecules (enzymes, transcriptional activators, and coactivators), with the end result a restoration of the cell's energy state. Serine-threonine liver kinase B1 (LKB1) is required for this phosphorylation; however, it is less clear whether changes in LKB1 activity specifically regulate it. Thus studies, predominantly in skeletal muscle, have suggested that LKB1 is constitutively active and that the conformational change induced by an increase in the AMP/ATP ratio increases phosphorylation at Thr172 by making AMPK resistant to the action of phosphatases (82). On the other hand, as is described in the text, activation of LKB1 and subsequently AMPK has been observed in various cells and tissues when silent information regulator T1 (SIRT1) is activated, and conversely, decreases in SIRT1 are associated with diminished LKB1 and AMPK activity (34, 48, 90). Not shown in the figure is that nutrient and O2 deprivation, increased energy expenditure (exercise), and various hormones typically initiate AMPK activation, whereas nutrient excess (e.g., high glucose) and other hormones (e.g., glucocorticoids) downregulate AMPK, leading to decreases in fatty acid oxidation and increases in lipid and protein synthesis (75). CaMKKβ, calcium/calmodulin kinase kinase-β; PP2A and PP2C, protein phosphatases 2A and 2C, respectively.