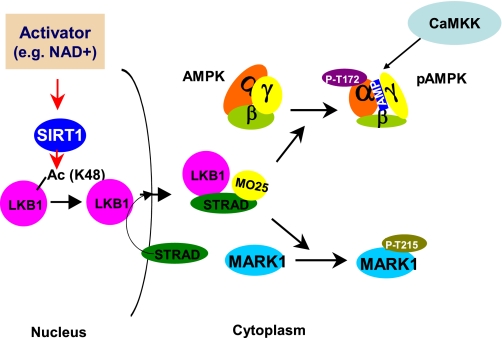
Fig. 4.
Proposed mechanism for activation of LKB1 and LKB1 target molecules by SIRT1 Activation of SIRT1 by genetic or pharmacological means in human embryonic kidney-293T cells (and presumably others) leads to deacetylation of Lys48 and possibly other key lysine residues on LKB1. This in turn enhances LKB1 binding to STE20-related adaptor protein (STRAD) and mouse embryo scaffold protein (MO25), which activates its kinase activity and leads to the phosphorylation of AMPK. The scheme assumes that SIRT1 is primarily nuclear and that LKB1 acetylation occurs in the nucleus and in some way enhances its movement to the cytoplasm (where it binds to STRAD). Since under some circumstances SIRT1 may be found in the cytoplasm, it is also possible that LKB1 acetylation could be an extranuclear event. In addition to AMPK, LKB1 phosphorylates and activates MARK1 and 12 other AMPK-related kinases (ARKs). CaMK kinase (CaMKK), which phosphorylates and activates AMPK even in the absence of LKB1, presumably would not activate the ARKs unless the increase in AMPK activity activated SIRT1 and, secondarily, LKB1 (adapted from Ref. 48).