Abstract
Background/Objective:
To determine the effects of spasticity on anthropometrics, body composition (fat mass [FM] and fat-free mass [FFM]), and metabolic profile (energy expenditure, plasma glucose, insulin concentration, and lipid panel) in individuals with motor complete spinal cord injury (SCI).
Methods:
Ten individuals with chronic motor complete SCI (age, 33 ± 7 years; BMI, 24 ± 4 kg/m2; level of injury, C6–T11; American Spinal Injury Association A and B) underwent waist and abdominal circumferences to measure trunk adiposity. After the first visit, the participants were admitted to the general clinical research center for body composition (FFM and FM) assessment using dual energy x-ray absorptiometry. After overnight fasting, resting metabolic rate (RMR) and metabolic profile (plasma glucose, insulin, and lipid profile) were measured. Spasticity of the hip, knee, and ankle flexors and extensors was measured at 6 time points over 24 hours using the Modified Ashworth Scale.
Results:
Knee extensor spasticity was negatively correlated to abdominal circumferences (r = −0.66, P = 0.038). After accounting for leg or total FFM, spasticity was negatively related to abdominal circumference (r = −0.67, P = 0.03). Knee extensor spasticity was associated with greater total %FFM (r = 0.64; P = 0.048), lower %FM (r = −0.66; P = 0.03), and lower FM to FFM ratio. Increased FFM (kg) was associated with higher RMR (r = 0.89; P = 0.0001). Finally, spasticity may indirectly influence glucose homeostasis and lipid profile by maintaining FFM (r = −0.5 to −0.8, P < 0.001).
Conclusion:
Significant relationships were noted between spasticity and variables of body composition and metabolic profile in persons with chronic motor complete SCI, suggesting that spasticity may play a role in the defense against deterioration in these variables years after injury. The exact mechanism is yet to be determined.
Keywords: Spasticity, Body composition, Resting energy expenditure, Metabolism, Spinal cord injuries, Paraplegia, Tetraplegia, Cardiovascular disease
INTRODUCTION
Spinal cord injury (SCI) results in complete or incomplete insult to the spinal cord with extensive skeletal muscle paralysis below the level of injury (1,2). The reduced neuromuscular activity after SCI coupled with a reduction in physical activity is accompanied by a dramatic deterioration in body composition and metabolic profile (3–8). In the first few months after injury, there is a rapid onset of skeletal muscle atrophy, increase of fat mass (FM), and decrease of fat-free mass (FFM) (4,7). For example, skeletal muscle cross-sectional area could be as low as 30% to 50% compared with able-bodied (AB) controls (9,10). Moreover, evidence has shown that %FM could easily exceed 30% in individuals with chronic SCI (8,11).
The loss of metabolically active lean tissue results in reduction of the basal energy expenditure in people with SCI (3,12,13). Energy expenditure and basal metabolic rate are lower even after adjusting for their FFM compared with healthy AB controls (12,13), predisposing them to a great risk of developing obesity (14,15). Individuals with SCI have more FM and lower energy expenditure compared with their monozygotic siblings without SCI (12). The changes in body composition after SCI are associated with numerous metabolic abnormalities, including glucose intolerance, insulin resistance (4–6), hyperlipidemia (5,6), and cardiovascular diseases (16). Recently, it was reported that 55% of individuals with SCI are at risk of developing metabolic syndrome (17). This could result in a shortened life span and health-related complications of socioeconomic burdens that negatively decrease the perceived quality of life after SCI and may interfere with their degree of independence and integration into the community (18).
The factors that influence the deterioration in energy expenditure, body composition, and metabolic profile after SCI are poorly understood. Understanding these factors may help to enhance the quality of life after SCI by providing appropriate medical and rehabilitation interventions. Spasticity is an upper motor neuron disorder that affects 70% of patients with chronic cervical and thoracic level injuries 1 year after injury (19). Spasticity is a complex phenomenon of exaggerated muscle tone, reflexes, and clonus that affects the skeletal muscles below the level of injury (19,20). Spasticity has often been viewed as a factor that can negatively impact functional progress after SCI (19,20). Despite its negative influence, spasticity could be viewed as expressing positive features (21,22). For example, spasticity has been shown to improve ambulation and peripheral circulation (23,24). Moreover, spasticity in the tail muscle of rats has proven to preserve the slow properties of skeletal muscle and attenuate the slow-to-fast transformation in myosin expression and myofiber atrophy that commonly occurs after SCI (25,26). Most recently, spasticity has been shown to defend against the process of skeletal muscle atrophy as early as 6 weeks after injury (21) and improve glucose homeostasis (22). Spastic thigh skeletal muscle size was 22% larger compared with the flaccid group (21). Moreover, glucose uptake was 3 times higher in the spastic limbs compared with controls (22).
Therefore, the purpose of this study was to investigate the protective effects of spasticity on whole body composition (FM and FFM) and metabolic profile (glucose tolerance, insulin sensitivity, and lipid profile) in chronic SCI. Additionally, the relationship between spasticity and resting metabolic rate (RMR) was studied. It is hypothesized that spasticity could positively attenuate the deterioration in body composition and metabolic profile in individuals with chronic SCI.
METHODS
Ten individuals with chronic traumatic motor complete SCI (8 men and 2 women; mean ± SD: age, 33 ± 7 years; body weight, 72 ± 11 kg; height, 176 ± 11 cm; level of injury, C6–T11; American Spinal Injury Association [ASIA] classification A, B) participated in the study. All participants were recruited from the university model SCI system and from Spasticity Clinic. Detailed descriptions for all procedures and risks of participation in this study were provided to each potential participant. All subjects gave written informed consent before the study. The institutional review board of The University of Michigan approved this study.
Participants were included if they were (a) men or women 18 to 45 years of age, with the maximum age chosen to avoid any confounding effects of the aging process on body composition and energy expenditures; (b) a minimum of 1 year after injury and had been maintained for 1 year on the same dose of oral baclofen, an antispasticity medication, to ensure stability of spasticity at the time of measurements; (c) the level of injury was C6 to T11. People with injury above C6 have limited hand functions and are dependent on others to prepare their meals or they potentially eat less. This could potentially result in lower body mass compared with other groups and influence their whole body composition. Lower motor neuron injury is typically found in those with injury below T11 and leads to flaccid paralysis of the involved skeletal muscles that ultimately affects body composition (21).
Participants were excluded from the study if they had hypertension, diabetes mellitus or elevated serum lipids, smoked or abused alcohol, had pressure ulcers greater than grade II, or were taking medications that affect metabolic profile, such as insulin. Individuals with body mass index (BMI) greater than 30 kg/m2 were excluded to avoid any confounding effects from increasing relative or absolute FM on the results. Additionally, BMI underestimates the percentage of FM in people with SCI (27). Etiologies for SCI other than trauma and other clinical populations with spasticity were excluded.
First Visit/Baseline Assessment
After undergoing a phone interview to determine eligibility, the 10 participants who met the study inclusion criteria were invited to the Health and Human Performance Laboratory located at the Department of Physical Medicine and Rehabilitation. The visit was timed at 7 to 10 days before admission to the General Clinical Research Center (GCRC) at the University of Michigan. On arrival, participants were asked to void their bladder and to undergo a general physical examination by a certified physician. The physical examination included measuring vital signs and a resting 12-lead electrocardiogram performed to rule out any pre-existing cardiac problems.
During the same visit, body weight (kg) and height (m) were measured to calculate their BMI (kg/m2). The weight of the participant was measured on a wheelchair scale (Tanita, Arlington Heights, IL, USA). After the participant moved to the testing table, the wheelchair was weighed empty and subtracted from the total weight. Height was measured in a supine position from the top of the head to the heel.
Because of a technical difficulty, a subset of our participants (n = 5) underwent a graded exercise test on an arm-crank ergometer (Monark Rehab Trainer 881E, Stockholm, Sweden) to measure their maximum aerobic capacity using a ParvoMedics (Sandy, UT) metabolic cart at a constant room temperature of 22°C. After calibration and assigning a nose clip, a sterilized mouth piece was secured with head mounting. The protocol consisted of 2-minute stages of exercise with 30-second intervals of rest to measure blood pressure. After 2 minutes of rest, the participant started pedaling at a resistance of 25 W and was asked to maintain a rate of 50 revolutions/min (RPM). Resistance was gradually increased by 25 W at the end of each 30-second rest interval. VO2 peak was considered when respiratory exchange ratio values were greater than 1.1 and they failed to maintain the assigned cranking rate.
Anthropometrics
Waist (WC) and abdominal circumferences were measured using an inelastic tape measure at the level of the narrowest part of the torso and at the umbilicus, respectively (14). The measurements were taken in sitting and supine positions, at the end of expiration, and without compressing the skin. Careful attention was made to positioning the tape measure at the same anatomical location when in sitting and supine positions.
Second Visit
The participants underwent a second visit to measure their body composition and metabolic profile. The timeline of the overnight 24-hour stay is presented in Figure 1. Participants reported to the GCRC at the University of Michigan hospital. After a routine physical examination, they underwent a body composition assessment using lunar dual energy x-ray absorptiometry (DEXA). After an overnight 12-hour fast, RMR was measured after lying for 30 minutes in a dark room. Data acquisition of the RMR was 20 minutes.
Figure 1.
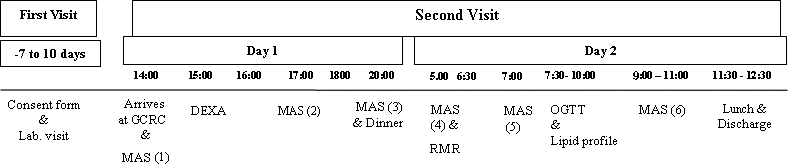
A frame of the timeline of the study during the first visit at the laboratory and the second visit at the GCRC. Time points are numbered MAS (1) to MAS (6).
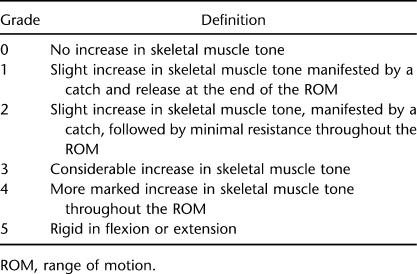
Modified Ashworth Scale
The Modified Ashworth scale (MAS) was used to evaluate spasticity for hip, knee, and ankle skeletal muscle flexors and extensors for both lower extremities (20,21,23). Spasticity was evaluated in a supine position by the same investigator (Table 1). The room temperature was held constant at 21°C to 24°C. Spasticity was measured over 24 hours during an overnight stay at 2:00 pm, 5:00 pm, and 8:00 pm (first day) and 5:00 am, 7:00 am, and 9:00 am (second day) (Figure 2). This approach was considered to account for fluctuations in spasticity previously reported (23). The average score of the 3 time points (2:00 pm, 5:00 pm, and 8:00 pm) was considered for statistical analysis because this represents the magnitude of spasticity on a daily basis under the effects of oral baclofen. The 3 time points that were measured in the morning overestimated the magnitude of spasticity, because participants were immobilized and delayed administration of their dosage of oral baclofen until concluding the metabolic study because oral baclofen is known to influence the metabolic profile in people with SCI (28). Spasticity was adjusted to the leg-FFM because spasticity was shown to explain portion of the variance in leg muscle size after SCI (21). The following equations were used to clarify how a single score was assigned to an individual muscle group (20).
| 1 |
| 2 |
where t = 1400, 1700, or 2000h.
 |
3 |
| 4 |
Table 1.
Six Grades of the MAS
Figure 2.
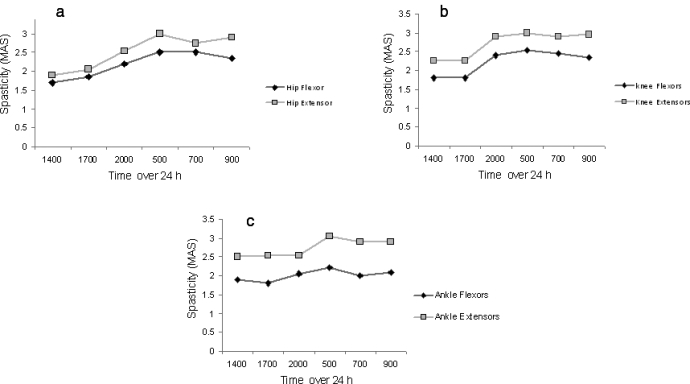
The average of spasticity scores of (a) hip, (b) knee, and (c) ankle flexors and extensors over the course of 24 hours. Hypertonicity was more in the extensor skeletal muscle groups compared with the flexors. A pattern of increased hypertonicity was detected in the morning because of immobilization and late administration of their oral baclofen dose.
Dual Energy X-Ray Absorptiometry
Body composition was measured using total body scans with a Lunar Prodigy Advance scanner (Lunar DPX, DEXA Scanner; Lunar, Madison, WI) (8,11). Regional (arms, legs, trunk, gynoid, android) and total body %FM and %FFM were measured (Figure 3). An index of FM to FFM was used to compare regional compartments. The coefficient of variability of repeated scans was less than 3%.
Figure 3.
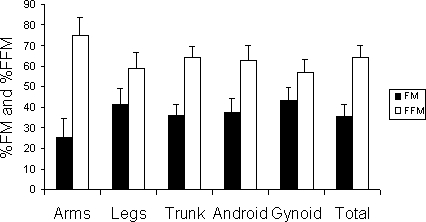
Mean ± SD of %FM and %FFM using lunar DEXA for the measurement of regional and total body composition.
Metabolic Profile
After an overnight fasting, participants underwent an array of examinations to measure their metabolic profile. At 6:00 am, basal metabolic rate and respiratory exchange ratio was measured by an indirect calorimetry unit (Vmax metabolic cart; SensorMedics Vmax, Yorba Linda, CA, USA) in a dark room located at the GCRC. Participants were asked to lie in a dark room for 20 to 30 minutes to attain a resting state, after which RMR was measured using a canopy-ventilated system for 20 minutes. The gases (Vco2 and Vo2) were collected to measure the respiratory exchange ratio and to calculate resting whole body rate of fat oxidation using the equation of Fryan (29).
Afterward, a Teflon catheter was inserted into an antecubital vein of 1 arm for blood sampling, and 4 mL/subject was sent for the analysis of triglycerides (TG), total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL). After allowing the blood sample to clot for 30 minutes, the blood was centrifuged at 3,000 RPM for 10 minutes, and the serum was transferred for analysis.
At 7:00 am, a standard 75-g oral glucose tolerance test (OGTT) was administered, and blood was drawn at 0 minute before and 30, 60, 90, and 120 minutes after administration. A 4-mL sample of blood was drawn at each time point to measure plasma glucose and insulin concentrations. All blood samples were sent to the Chemistry Pathology Laboratory for analysis. The plasma concentrations of TGs and glucose concentrations were determined using commercially available colorimetric assays (Sigma, Wako, St. Louis, MO, USA and Thermo DMA, Arlington, TX, USA, respectively). The plasma insulin concentration was measured using a commercially available radioimmunoassay kit (Linco Research, St. Charles, MO, USA). Insulin sensitivity was determined using the Matsuda and Defronzo formula: Insulin sensitivity = 10.000/sq. root (Fasting plasma glucose × fasting plasma insulin) × (mean OGTT glucose concentration × mean OGTT insulin concentration) (30). Additionally, the HOMA index was calculated as the product of the fasting plasma insulin level (µU/mL) and the fasting plasma glucose level (mmol/L), divided by 22.5. Finally, the areas under the curve (AUC) for plasma glucose and insulin were computed by integration with trapezoidal rule.
Statistical Analysis
Considering the ordinal nature of MAS, Spearman ρ correlation was used to determine the relationship between spasticity, body composition, RMR, and metabolic profile. The Pearson correlation was used to determine the relationship among body composition, RMR, and metabolic profile variables. A continuous design was used because the current population is characterized by many confounding factors, and matching between 2 groups is extremely difficult. Partial correlations were used to adjust for time since injury and oral baclofen dose for all the studied variables. Repeated-measure analysis of variance was used to test for differences among the indices of FM to FFM of arms, legs, trunk, and total body. All values are presented as mean ± SD, and statistical significance was set at a level of P < 0.05.
RESULTS
The time since injury was 11 ± 7 years (range, 2–22 years). BMI of the subjects was 24 ± 4 kg/m2. Vo2 peak was 0.92 ± 0.3 L/min (13.05 ± 4.5 mL/kg/min), suggesting low exercise tolerance.
Spasticity
The outcomes of MAS of the 6 skeletal muscle groups over 24 hours are presented in Figure 2. The sum of hip, knee, ankle flexor and extensor spasticity was 4.5 ± 2 and 6 ± 3, respectively (P = 0.001). Spasticity scores of the hip flexors vs extensors were 2.1 ± 0.9 vs 2.4 ± 0.9 (P = 0.08), knee flexors vs exstensors were 2.0 ± 0.8 vs 2.6 ± 1.0 (P = 0.005), and ankle flexors vs exstensors were 2.0 ± 0.8 vs 2.5 ± 1.0 (P = 0.055).
Spasticity and Anthropometrics
The average of the lower extremity flexor or extensor spasticity was not related to WCs and abdominal circumferences. However, there were trends toward a negative association between knee flexor (r = −0.60, P = 0.064) or extensor spasticity (r = −0.57, P = 0.08) and abdominal circumference in the lying position. The average of extensor spasticity (hip, knee, and ankle) adjusted to total body FFM or leg FFM was negatively related to abdominal circumferences (r = −0.67, P = 0.03). Knee extensor spasticity was negatively correlated to abdominal circumferences (r = −0.66, P = 0.038). There were trends toward negative associations with WC (r = −0.56 and −0.6), but they did not attain significance (P > 0.5).
Spasticity and Body Composition
Total body composition assessment showed that %FFM and %FM were 64 ± 6% and 36 ± 6%, respectively (Figure 3). Increasing knee extensor spasticity was associated with a reduction in the %FM of the gynoid region (r = −0.65, P = 0.047; Figure 4a), trunk (r = −0.65, P = 0.039), and total body (r = −0.65, P = 0.03; Figure 4b), but not leg %FM. Additionally, increasing knee flexor spasticity was associated with reduction in trunk and total %FM (r = −0.73, P = 0.016 and r = −0.64, P = 0.048, respectively). Knee extensor spasticity was directly associated with an increase in %FFM in the gynoid region and total body (r = 0.63 and 0.65, respectively, P < 0.03). Additionally, knee flexor spasticity was directly associated with an increase in % FFM in the trunk (r = 0.73, P = 0.01) and total body (r = 0.64, P = 0.048).
Figure 4.
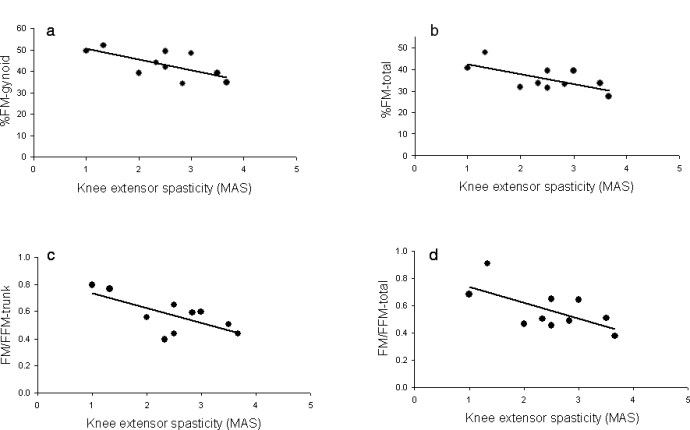
Inverse relationships between knee extensors spasticity and %FM in (a) gynoid (r = −0.65, P = 0.047) and (b) total body (r = −0.65, P = 0.03) and indices of FM to FFM in (c) trunk (r = −0.69, P = 0.025) and (d) total body (r = −0.65, P = 0.04).
The ratio of FM/FFM was 0.73 ± 0.23, 0.36 ± 0.20, 0.57 ± 0.13, and 0.56 ± 0.15 in leg, arm, trunk, and total body composition, respectively (P = 0.0001). Pairwise comparisons showed significant differences between the ratio in legs and arms (P = 0.0001) and arms and trunk (P = 0.038), suggesting an increase in the fat mass in legs and trunk compared with the arms. The average of knee extensor spasticity was negatively related to the ratio of FM/FFM of legs (r = −0.56, P = 0.08), trunk (r = −0.69, P = 0.025), and total body (r = −0.65, P = 0.04; Figure 4c and d).
Spasticity and Metabolic Profile
The RMR was 1,256 ± 231 kcal/d. The respiratory exchange ratio was 0.76 ± 0.05 and fat oxidation was 71 ± 20 g/min. Increased FFM (kg) was associated with higher RMR (r = 0.89; P = 0.0001; Figure 5); however, spasticity did not influence RMR. Knee extensor and flexor spasticity adjusted to leg FFM showed a strong association with fat oxidation (r = 0.46 and 0.57; P = 0.08).
Figure 5.
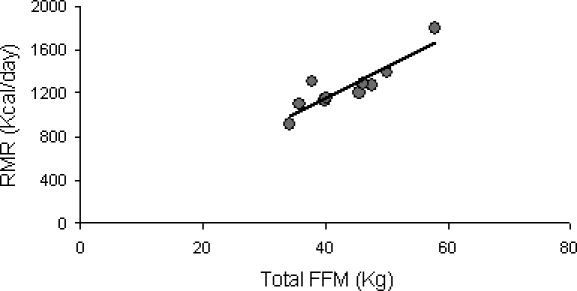
The relationship between FFM-total body (kg) and RMR (kcal/d; r = 0.89; P = 0.0001).
Fasting plasma glucose and insulin were 87 ± 10 mg/dL and 13 ± 3 µm/mL, respectively. After administering the OGTT, plasma glucose increased to 132 ± 28, 121 ± 30, 113 ± 27, and 117 ± 31 mg/dL at 30, 60, 90, and 120 minutes, respectively. Plasma insulin increased to 106 ± 51, 100 ± 49, 72 ± 41, and 74 ± 49 µm/mL at the corresponding time points. The Homeostatic Model of Assessment index was 2.8 ± 0.8, and insulin sensitivity using the Mostuda and Defronzo formula was 3.8 ± 1.8. The AUCs for glucose and insulin were 672 ± 127 and 408 ± 171, respectively. A trend of reduction in plasma glucose at T120 was associated with an increase in %FFM-total (r = −0.59, P = 0.06). %FFM leg was associated with a reduction in glucose AUC (r = −0.56, P = 0.08). An increase in hip extensor spasticity correlated with a reduction in plasma insulin concentration at T30 (r = −0.63, P = 0.049). Additionally, an increase in knee extensor spasticity associated with a reduction in plasma insulin concentration at T60 (r = −0.68, P = 0.03). Spasticity did not seem to influence insulin sensitivity using the Matsuda and Defronzo formula or the HOMA index. Knee extensor or flexor spasticity did not seem to influence AUCs of plasma glucose and insulin. However, after adjusting to total FFM, extensor spasticity was negatively correlated to AUCs of plasma glucose and insulin (r = −0.64, P = 0.04).
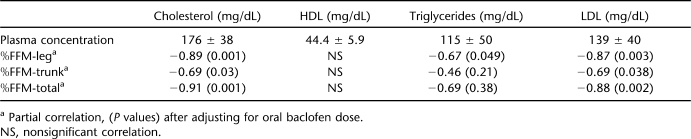
The outcomes of lipid profile are presented in Table 2. An increase in %FFM-total was associated with a reduction in plasma cholesterol (r = −0.79; P = 0.006) and LDL (r = −0.75; P = 0.01). %FFM-trunk and leg was associated with a reduction in plasma cholesterol (r = −0.65, P = 0.042 and r = −0.64, P = 0.045, respectively) and LDL (r = −0.59, P = 0.06 and r = −0.61, P = 0.062, respectively). Partial correlations after adjusting for oral baclofen are presented (Table 2). Extensor spasticity was not related to any of the outcomes of the lipid panel. However, knee extensor spasticity was negatively related to LDL (r = −0.56, P = 0.049). After adjusting knee extensor spasticity for leg FFM, this relationship was more pronounced (r = −0.70, P = 0.025).
Table 2.
Outcomes of the Lipid Profile After Overnight Fasting at the GCRC (n = 10)
DISCUSSION
The tonic activity associated with spasticity may have a protective effect against deterioration in body composition, energy expenditure, and metabolic profile in individuals with motor complete SCI. Those with increased spasticity in large muscle groups have less FM and maintain high FFM. Spasticity per FFM is inversely related to abdominal circumference. Moreover, spasticity improves glucose homeostasis, insulin sensitivity, and lipid profile by primarily maintaining FFM. Additionally, maintenance of FFM has been shown to positively influence the RMR and hence the basal metabolic profile.
The findings support the observation that spasticity may attenuate skeletal muscle atrophy early after incomplete SCI and in people with chronic stroke (21,31). Spasticity could mimic isometric contraction or low tension resistance training in maintaining lean mass. Franzoi et al (32) showed that individuals with MAS 3 developed more torque output compared with those with MAS 1 or 2 or AB controls. Similar to aerobic or resistance training, the periodic tension of spasticity has no preferential site specific on subcutaneous adipose tissue. There were strong relationships between spasticity and FM (total, trunk, gynoid) but not leg FM. In men, 20 weeks of aerobic training altered body fatness but the induced fat loss does not deplete subcutaneous fat at a specific site (33). In women, 5 weeks of resistance training caused increased muscle mass and reduction in total fat mass, with no change in the thigh fat mass (34).
Previous reports documented a positive relation between insulin growth factors and the body composition profile (35,36). It is plausible that spasticity enhanced the expression of endogenous growth hormone and/or the potency of insulin growth factors (28), thus improving body composition profile. Growth hormone response is blunted in individuals with chronic SCI (37); whether spasticity enhances the quality of growth hormone sensitivity needs to be further addressed. Another explanation is the ability of spastic muscle to attenuate the shift in slow to fast muscle fiber transformation (25,26); slow fibers have a higher mitochondrial concentration that may be responsible for the increased fat oxidation observed in this study. However, this may not be universal finding because hind limb suspension in rats seems to reduce mitochondrial volume in all fiber types (38).
Periodic recruitment of large skeletal muscle mass could result in high energy expenditure and defend against increase in FM after SCI. The involuntary activity associated with spasticity may have resulted in increasing the noncontractile ATP costs because of the activity of Na+-K+-ATPase and Ca2+-ATPase. In accordance with recent findings (39), spasticity was not directly related to the RMR. However, successfully maintaining FFM resulted in increased RMR, respiratory exchange ratio, and substrate utilization, as documented by increased fat oxidation. Therefore, therapeutic interventions that can attenuate skeletal muscle atrophy and promote hypertrophy are highly recommended to balance the disturbance in energy expenditure after SCI (40). Knee extensor spasticity positively decreases the ratio between FM to FFM in legs, trunk, and whole body. The increase in FM relative to FFM can be simply attenuated by provoking spasticity in both lower extremities. The index of FM/FFM in the arms represents a favorable norm that could be used to determine the deviation in other regional compartments, especially for those with low cervical lesion and individuals with paraplegia.
Negative relationships were observed between spasticity and WC and abdominal circumference. Both WC and abdominal circumference have been used as indicators of abdominal fat accumulation in healthy and clinical populations. It would have been interesting to measure visceral adiposity as determined by ultrasound or magnetic resonance imaging techniques. However, circumferential measurements have been used as an index for developing metabolic syndrome (41) and visceral adiposity accumulation after SCI (42). The relationships became negative after considering the %FM in the trunk and gynoid region, indicating that increasing spasticity in lower extremities may contribute to reduce abdominal FM. The relationship was inversely related after adjusting spasticity to FFM in the lower extremities, suggesting that tension per unit FFM could play a role in reducing abdominal fat. The speculation was supported by the strong relationship between FFM and RMR; this may indirectly contribute to caloric expenditure and reduction in FM.
Skeletal muscle oxidative capacity is impaired in individuals with SCI, predisposing them to further weight again and developing type 2 diabetes mellitus (5,6). This fact is confirmed by their low aerobic capacity and the high percentage of fast twitch fibers distributed in the paralyzed skeletal muscles (25,26). This may result in impaired substrate utilization and lead to insulin resistance commonly seen in obese and elderly individuals (43). These findings may support the notion that spasticity may enhance glucose uptake and improve insulin sensitivity by maintaining FFM. In motor complete SCI, spasticity could counteract the risk of developing type II diabetes mellitus by increasing glucose uptake 3 times higher compared with AB controls (22). It is unclear how spasticity improves glucose homeostasis; however, it has been suggested that spasticity could have a significant role through an insulin-independent mechanism (22). It is possible that spastic muscle fibers triggers Ca++ release and stimulates translocation of GLUT-4. Previously, we documented that spasticity could help to reduce intramuscular fat, which is proven to impair insulin sensitivity (9,21).
Persons with SCI have lower serum HDL cholesterol levels than AB controls (7,44). Serum total and LDL cholesterol, as well as TGs, were all higher in persons with paraplegia compared with controls because of disturbances in the autonomic nervous system and low aerobic capacity (44). This may impose an atherogenic risk of developing cardiovascular diseases and may explain the higher incidence in SCI (∼228%) compared with healthy controls (4). Increasing physical activity improves the lipid profile and HDL in individuals with SCI (44,45,46). Spasticity has been shown to benefit the overall picture of lipid profile by reducing cholesterol, LDL, and TGs. The findings may suggest that the use of spasticity as an inherent tool to exercise the paralyzed muscle may improve the overall picture of lipid profile. This can be accomplished by varying the dose of oral baclofen over the course of treatment. Therefore, adjusting for oral baclofen is crucial when managing spasticity in individuals with dyslipidemia.
The primary weakness of this study is the failure to account for baseline variations in body composition and metabolic profile in this population. Body composition may vary widely among individuals with SCI as a result of the level of injury, years after injury, and extent of physical activity; therefore, it is unlikely that spasticity can explain most of the variance in body composition after SCI. The current observations about spasticity, body composition, and metabolic profile are correlative, and direct inferences are unlikely to be made. However, the study sheds light on factors that need to be further addressed on the mechanistic role of spasticity after SCI, lending credence to a longitudinal design to account for baseline variations. This study followed strict inclusion criteria to ensure a homogeneous small sample size. Smoking, different antispasmodic medications, BMI > 30 kg/m2, and other chronic complications were primary factors that warranted exclusion from the study. Despite quantifying spasticity over a 24-hour stay, the use of MAS is considered a subjective tool, and objective measures are highly recommended for future studies. Therefore, it is crucial to evaluate extensor spasticity separated from the flexor one. The overall effects of lower extremity spasticity (extensor + flexor) may not accurately reflect the exact relationships on the examined variables, because flexor spasticity may dilute the effects of extensor spasticity (Figure 3).
CONCLUSION
Considering the well-established negative effects of spasticity, this study showed evidence that individuals with motor complete SCI may have desirable effects with regard to their body composition and metabolic profile in response to spastic skeletal muscles. Spasticity may be used as an exercise tool to develop periodical tension and protect against other health-related complications. Potential clinical consideration should be given to balance functional and quality of life issues related to muscle spasticity with those related to soft tissue, metabolic, and long-term cardiovascular outcomes.
Acknowledgments
We thank all our participants and Dr Denise Tate for providing guidance during the course of the study.
Footnotes
This work was supported through funding of Scholarship to Advance Research in Spasticity (STARS) Foundation, National Institute on Disability and Rehabilitation Research (NIDRR) Grant H133P030004, and the General Clinical Research Center (NIH Funding to the University of Michigan).
REFERENCES
- Sekon LH, Fehlings MG. Epidemiology, demographics and pathophysiology of acute spinal cord injury. Spine. 2001;26(24 suppl):S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. Available at: http://main.uab.edu. Accessed January 14, 2009.
- Buchholz AC, McGillivray CF, Pencharz PB. Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res. 2003;11(4):563–570. doi: 10.1038/oby.2003.79. [DOI] [PubMed] [Google Scholar]
- Kocina P. Body composition of spinal cord injured adults. Sports Med. 1997;23(1):48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–277. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30(10):697–703. doi: 10.1038/sc.1992.136. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95(6):2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Modlesky CM, Bickel CS, Slade JM, Meyer RA, Kureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol. 2004;96(2):561–565. doi: 10.1152/japplphysiol.00207.2003. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Wang J, Pierson RN., Jr The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev. 2004;41(1):1–8. doi: 10.1682/jrrd.2004.01.0001. [DOI] [PubMed] [Google Scholar]
- Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7(6):635–639. doi: 10.1097/00075197-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43(9):513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Gater DR., Jr Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12(4):1–7. doi: 10.1310/sci1204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466–476. doi: 10.1038/sj.sc.3102161. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Widman LM, Abresch RT, et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30(suppl 1):S127–S139. doi: 10.1080/10790268.2007.11754591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35(12):809–813. doi: 10.1038/sj.sc.3100501. [DOI] [PubMed] [Google Scholar]
- Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80(12):1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury [published correction appears in Spinal Cord. 2008;46(12):825] Spinal Cord. 2008;46(2):96–102. doi: 10.1038/sj.sc.3102087. [DOI] [PubMed] [Google Scholar]
- Bennegard GM, Karlsson AK. Higher glucose uptake in paralysed spastic leg. Spinal Cord. 2008;46(2):103–106. doi: 10.1038/sj.sc.3102083. [DOI] [PubMed] [Google Scholar]
- Sköld C. Spasticity in spinal cord injury: self- and clinically rated intrinsic fluctuations and intervention-induced changes. Arch Phys Med Rehabil. 2000;81(2):144–149. doi: 10.1016/s0003-9993(00)90132-1. [DOI] [PubMed] [Google Scholar]
- Young RR, Woolsey RM, editors. Diagnosis and Management of Disorders of the Spinal Cord. Philadelphia, PA: WB Saunders Company; 1995. [Google Scholar]
- Harris RL, Bobet J, Sanelli L, Bennett DJ. Tail muscles become slow but fatigable in chronic sacral spinal rats with spasticity. J Neurophysiol. 2006;95(2):1124–1133. doi: 10.1152/jn.00456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RL, Putman CT, Rank M, Sanelli L, Bennett DJ. Spastic tail muscles recover from myofiber atrophy and myosin heavy chain transformations in chronic spinal rats. J Neurophysiol. 2007;97(2):1040–1051. doi: 10.1152/jn.00622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Legge M, Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003;84(7):1068–1071. doi: 10.1016/s0003-9993(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Kirshblum SC, Morrison NG, Cirnigliaro CM, Zhang RL, Spungen AM. Effect of low-dose baclofen administration on plasma insulin-like growth factor-I in persons with spinal cord injury. J Clin Pharmacol. 2006;46(4):476–482. doi: 10.1177/0091270006286641. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Pang MY, Ashe MC, Eng JJ. Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporos Int. 2007;18(9):1243–1252. doi: 10.1007/s00198-007-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoi AC, Castro C, Cardone C. Isokinetic assessment of spasticity in subjects with traumatic spinal cord injury (ASIA A) Spinal Cord. 1999;37(6):416–420. doi: 10.1038/sj.sc.3100849. [DOI] [PubMed] [Google Scholar]
- Després JP, Bouchard C, Tremblay A, Savard R, Marcotte M. Effects of aerobic training on fat distribution in male subjects. Med Sci Sports Exerc. 1985;17(1):113–118. [PubMed] [Google Scholar]
- Eliakim A, Burke GS, Cooper DM. Fitness, fatness, and the effect of training assessed by magnetic resonance imaging and skinfold-thickness measurements in healthy adolescent females. Am J Clin Nutr. 1997;66(2):223–231. doi: 10.1093/ajcn/66.2.223. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17(5):481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Gomez JM. Serum leptin, insulin-like growth factor-I components and sex-hormone binding globulin. Relationship with sex, age and body composition in healthy population. Protein Pept Lett. 2007;14(7):708–711. doi: 10.2174/092986607781483868. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Flanagan S, Zhong YG, Alexander LR, Tsitouras PD. Blunted growth hormone response to intravenous arginine in subjects with a spinal cord injury. Horm Metab Res. 1994;26(3):152–156. doi: 10.1055/s-2007-1000798. [DOI] [PubMed] [Google Scholar]
- Takekura H, Yoshioka T. Ultrastructural and metabolic profiles on single muscle fibers of different types after hindlimb suspension in rats. Jpn J Physiol. 1989;39(3):385–396. doi: 10.2170/jjphysiol.39.385. [DOI] [PubMed] [Google Scholar]
- Yilmaz B, Yasar E, Goktepe S, et al. Basal metabolic rate and autonomic nervous system dysfunction in men with spinal cord injury. Obesity (Silver Spring) 2007;15(11):2683–2687. doi: 10.1038/oby.2007.320. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80(4):394–396. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Clasie J, Gater DR. Waist circumference determines the risk of obesity and metabolic syndrome in spinal cord injury [Presentation 4] Arch Phys Med Rehabil. 2006;87(10):e7–e8. [Google Scholar]
- Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87(3):600–607. doi: 10.1093/ajcn/87.3.600. [DOI] [PubMed] [Google Scholar]
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA San Antonio Metabolism Study. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47(1):31–39. doi: 10.1007/s00125-003-1263-9. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Figoni SF. High density lipoprotein cholesterol in individuals with spinal cord injury: the potential role of physical activity. Spinal Cord. 1999;37(10):685–695. doi: 10.1038/sj.sc.3100917. [DOI] [PubMed] [Google Scholar]
- de Groot S, Dallmeijer AJ, Post MW, Angenot EL, van der Woude LH. The longitudinal relationship between lipid profile and physical capacity in persons with a recent spinal cord injury. Spinal Cord. 2008;46(5):344–351. doi: 10.1038/sj.sc.3102147. [DOI] [PubMed] [Google Scholar]
- El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord. 2005;43(5):299–305. doi: 10.1038/sj.sc.3101698. [DOI] [PubMed] [Google Scholar]