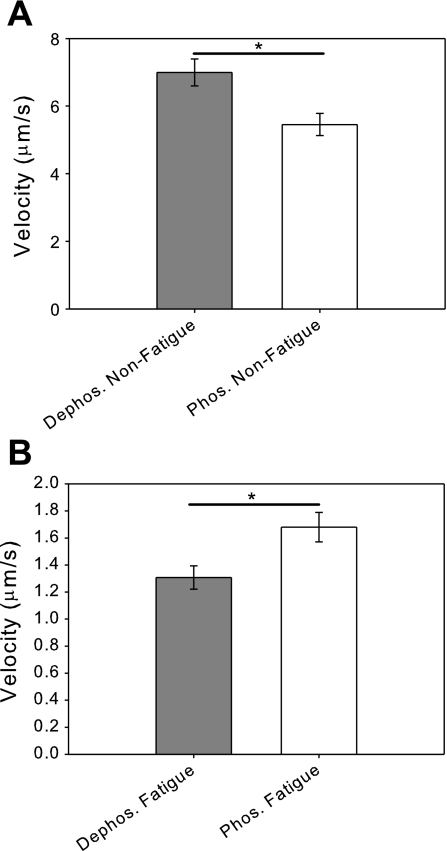
Fig. 1.
Fatigue-like conditions reduce actin filament sliding velocity. A: comparison of the unloaded sliding velocities of regulatory light-chain dephosphorylated and phosphorylated myosins under basal conditions. The velocity of phosphorylated myosin (V = 5.5 ± 0.3 μm/s) is significantly reduced compared with the dephosphorylated myosin (V = 7.0 ± 0.4 μm/s ; *P < 0.005). B: comparison of the unloaded sliding velocities of dephosphorylated and phosphorylated myosins under fatigue-like conditions shows that fatigue-like conditions cause a reduction of sliding velocity for both dephosphorylated and phosphorylated myosins. In contrast to the results under basal conditions, the velocity of phosphorylated myosin (V = 1.7 ± 0.1 μm/s) is significantly increased compared with dephosphorylated myosin (V = 1.3 ± 0.1 μm/s; *P < 0.02).