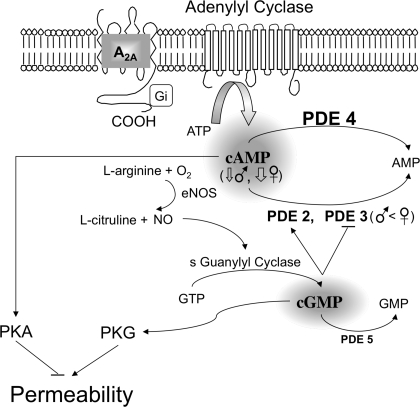
Fig. 6.
Schematic of the mechanisms whereby PDEs mediate endothelial barrier integrity. PDE isoforms 2–5 are expressed constitutively in primary EC derived from rat skeletal muscle microvessels. PDE4 and -5 hydrolyze intracellular cAMP and cGMP, respectively. Both PDE2 and PDE3, hydrolyzing cAMP, are the critical molecules bridging between cGMP and cAMP. Their activity creates the cross talk, resulting in loosening and tightening the endothelial barrier via PKG and PKA pathways, respectively. PDE2 is activated, while PDE3 is inhibited by cGMP. Given that XX EC express higher levels of PDE3 and comparable levels of PDE2, -4, and -5 relative to XY EC, the intracellular levels of cAMP are expected to be elevated greatly in XX EC compared with XY EC. This appears to be the case only when the system is activated (e.g., when cilostazol is given to block PDE3-mediated cAMP degradation). Consequently, while basal Ps did not differ in monolayers of XX and XY EC, the decrease in Ps in response to cilostazol was greater in XX than XY EC. eNOS, endothelial nitric oxide synthase.