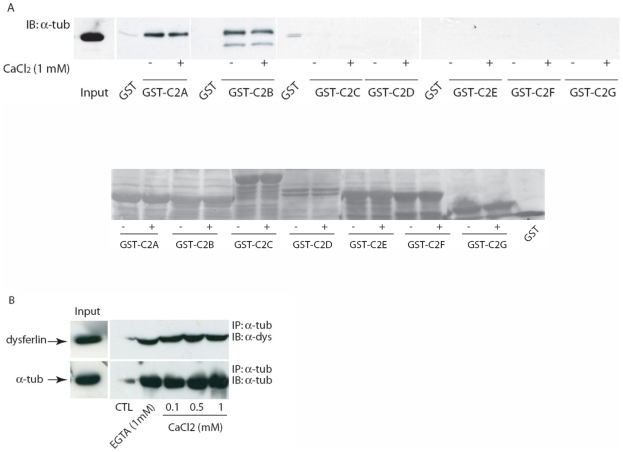
Figure 5. Alpha-tubulin interacts with dysferlin in a calcium-independent manner.
A. Upper panel: Myoblast cell extracts were incubated with GST alone or the various GST-dysferlin C2 domain fusion proteins precoupled to glutathione-Sepharose 4B beads in the absence (−) or presence (+) of 1 mM calcium. The bound proteins were separated on SDS-PAGE followed by Western blot analysis using anti-alpha-tubulin antibody. Lower panel: nitrocellulose membrane of GST-dysferlin C2 domains with adsorbed proteins from the cell extract stained with ponceau red. B. Co-immunoprecipitation of alpha-tubulin and dysferlin with anti-alpha-tubulin antibody from mouse skeletal muscle homogenate in the presence of increasing calcium concentrations. Proteins were separated and detected with anti-alpha-tubulin and anti-dysferlin antibodies. As a control (CTL), protein A-Sepharose beads were incubated with muscle homogenate in the absence of anti-alpha-tubulin antibody.