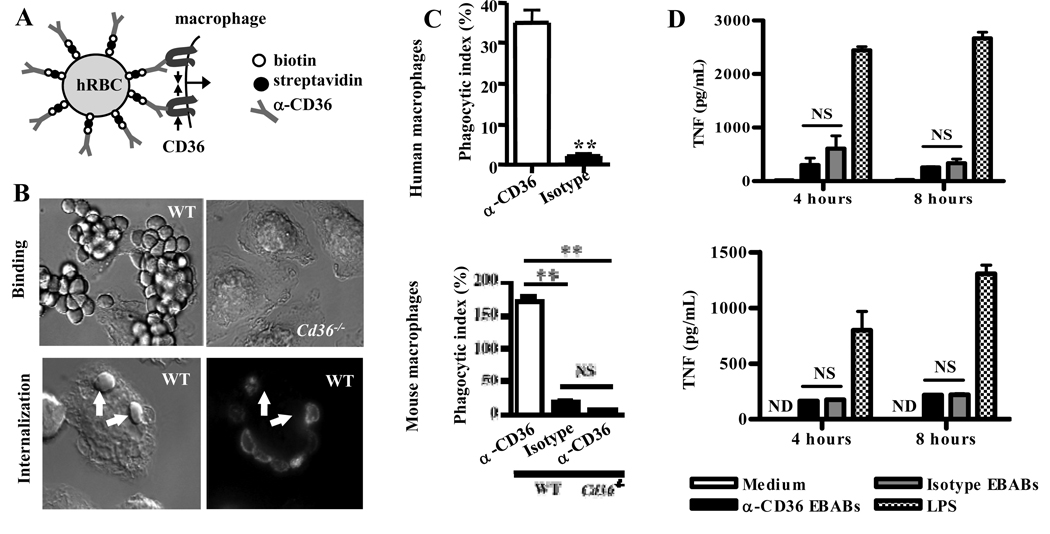
Fig. 2. CD36-mediated phagocytosis of large particles does not result in secretion of proinflammatory cytokines.
(A) Schematic diagram of human and mouse α-CD36 EBABs. α-CD36 antibodies conjugated to human red blood cells (RBC) via biotin and streptavidin crosslink CD36 on the macrophage surface, resulting in internalization. (B) Differential interference contrast images of α-CD36 EBABs binding to wild-type and Cd36−/− murine macrophages (upper panels) and internalization by wild-type macrophages (lower panels). After an internalization assay, EBAB uptake was confirmed by staining bound EBABs with a fluorophore-conjugated antibody; following a hypotonic lysis, the ghosts of lysed bound EBABs appeared stained, whereas internalized EBABs (indicated by arrows) did not. (C) Human macrophages (top) and wild-type and Cd36−/− murine macrophages (bottom) were incubated with α-CD36 EBABs and EBABs prepared with an isotype-matched control antibody, and phagocytosis was assessed. ** indicates p<0.01 by Student’s t-test (human data) or one-way ANOVA with Bonferroni post-tests (murine data). (D) α-CD36 EBABs or isotype control EBABs were incubated with human (top) or murine (bottom) macrophages at 37°C for 4 and 8 hr, and TNF in supernatants was measured by ELISA. NS, not significant by Student’s t-test. Results are representative of three independent experiments.