Table 1.
Comparison of the yield of the coupling of aryl halides with 4-methoxythiophenol after 24 h.
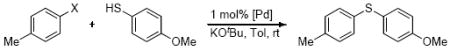 | |||
|---|---|---|---|
| Entry[a] | X | [Pd] | Yield (%)[b] |
| 1 | Br | Pd(OAc)2, CyPF-tBu | 0 |
| 2 | Br | Pd(dba)2, CyPF-tBu | 0 |
| 3 | Br | Pd(CyPF-tBu)(p-tolyl)(Br) | 99 |
| 4 | Cl | Pd(OAc)2, CyPF-tBu | 0 |
| 5 | Cl | Pd(dba)2, CyPF-tBu | 0 |
| 6 | Cl | Pd(CyPF-tBu)(p-tolyl)(Br) | 99 |
Reaction conditions: ArX (1 mmol), thiol (1 mmol), KOtBu (1.4 mmol), toluene (1.5 mL), 24 h.
Determined by GC using dodecane as internal standard.
