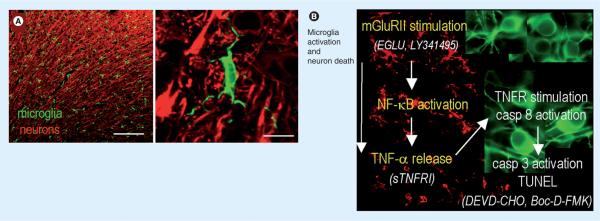
Figure 4. Summary of findings and model of the ischemic penumbra.
(A) Representative confocal micrographs of the healthy rat cortex, showing the dense network of ramified microglia whose processes are in close apposition to the neurons. Scale bars: left, 200 μm; right, 10 μm. A higher-magnification image shows a typical microglial cell with processes abutting the neuronal cell body (near top) and wrapping around the axonal processes. Microglia are labeled with a rabbit polyclonal antibody against a membrane protein, IBA1, and an Alexa 488-conjugated goat anti-rabbit secondary antibody (green). Neurons are labeled with a mouse monoclonal MAP2 antibody and Cy3-conjugated goat antimouse secondary antibody (red). (B) After a stroke, an inflammatory response develops over time, microglia become activated (their processes retract and they migrate to the damage site), and neuronal damage propagates into the surrounding tissue, the ischemic `penumbra'. Note that the colors are reversed from (A); microglia (red) are labeled with IBA1 antibody and a Cy3-conjugated secondary antibody, whereas rat cortical neurons (green) are labeled with microtubule-associated protein-2 and a FITC-conjugated secondary antibody. Author's interpretation [49]: “In these representative confocal micrographs from the rat brain after a stroke, microglia (green) are ramified and densely distributed in the uninjured contralateral hemisphere (top image), but retract their processes and eventually become rounded up as they progressively activate on the damaged ipsilateral side (bottom two images). In this study, using an in vitro model of the stroke penumbra, we identified several events: glutamate, released by the oxygen–glucose deprivation-stressed neuron–astrocyte cultures, reached sufficient concentrations to stimulate microglial mGluRIIs; this stimulation was blocked by the selective mGluRII antagonists, EGLU or LY341495; microglial activation was accompanied by activation of NF-κB, not p38 MAPK; activated microglia produced and released TNF-α; TNF-α interacted with the target neurons, activating their p55/TNF1 receptors, as judged by activation of caspase-8. Neurotoxicity was reduced by scavenging TNF-α with sTNFR1; the target neurons died by apoptosis, as judged by caspase-3 activation and TUNEL; caspase 3 activation was required for excess neuron killing, because it was prevented by the caspase-3 inhibitor, DEVD-CHO, and by the broad-spectrum caspase inhibitor, Boc-D-FMK.”
mGluR: Metabatropic glutamate receptor II; sTNFR1: Soluble TNF-α receptor 1; TNFR: TNF receptor.
Adapted with permission from [49].