Abstract
Context: Adipokines have been linked to bone phenotypes recently, but with conflicting results. Few such studies have been conducted in adolescents.
Objective: The aim of the study was to examine the associations of adiponectin and leptin with multiple bone phenotypes in Chinese adolescents and estimate the genetic contribution to these associations.
Design and Setting: This was a cross-sectional study conducted in rural China.
Participants: A total of 675 males and 575 females aged 13–21 yr were included.
Outcome Measures: Fat mass (FM), lean mass (LM), bone area (BA), bone mineral content (BMC), cross-sectional area (CSA), and section modulus (SM) were measured by dual-energy x-ray absorptiometry. Plasma adipokine concentration was determined using sandwich immunoassays.
Results: Adiponectin was inversely associated with all BMCs in males (P < 0.01), but not in females, after adjusting for LM, body weight, or BMI singly, or for LM and FM simultaneously. No such relationships were observed for CSA or SM in both genders. Leptin was inversely associated with all BAs, total-hip BMC, CSA, and SM in both genders, when adjusting for body weight or BMI. These associations, except for whole-body BA and lumbar spine BA in females, disappeared when simultaneously adjusting for LM and FM. By Cholesky decomposition models using twin design, significant genetic correlations were detected between adiponectin and total-hip BMC in males and between leptin and total-hip BMC in both genders.
Conclusions: We demonstrated that adiponectin and leptin were inversely associated with adolescent bone phenotypes but showed differential associations by gender, type of bone phenotypes, and adjustment of FM. This study also suggested that adipokines and bone phenotypes may share a common set of genes.
Adiponectin and leptin were inversely associated with bone phenotypes, but appeared to have differential associations by gender, bone material versus geometry parameters, and adjustment of fat mass.
Recent studies have shown that obesity negatively affects bone health in both adults (1) and adolescents (2,3). However, the underlying biological mechanism by which excess adipose tissue affects bone health is unclear. Adipocyte-derived hormones or adipokines, including leptin and adiponectin, have been proposed to be a link between adipose tissue and bone. For instance, higher bone mass has been observed in leptin signaling-deficient mice (4). Animal studies have suggested that leptin acts at the level of the hypothalamus on various neurons, inducing two molecular cascades–one cascade inhibiting osteoblast formation via a “molecular clock,” and the other promoting bone resorption through increased receptor activator of nuclear factor-κB ligand (RANKL) expression (5,6). Recently, it was shown that human osteoblasts and precursors express adiponectin and its receptors (7). Moreover, an in vitro study showed that recombinant adiponectin could increase osteoclast formation indirectly through stimulating RANKL and inhibiting osteoprotegerin production in osteoblast (8). Taken together, one would speculate a negative effect of adiponectin and leptin on bone phenotypes.
To date, human studies on the relationship between adipokines and bone phenotypes have been inconclusive. Most studies were conducted in adults: some found a positive association (9,10), whereas others found a negative association (11,12). There are only a few studies examining the relationship of adipokines with bone mass in children and adolescents (13,14,15,16,17,18); most of these studies consist of fewer than 200 subjects and focus mainly on leptin (15,16,17,18).
In light of growing obesity epidemics in children and adolescents worldwide, further studies on adipokines and bone phenotypes in children and adolescents are clearly warranted. First, although osteoporosis is typically diagnosed in late adulthood, there is an increasing recognition that the origin of osteoporosis can be traced back to childhood and adolescence (19). Factors or conditions that can alter bone formation or enhance bone resorption during adolescence will greatly affect the risk of osteoporotic fracture later in life (20). Second, both bone material properties and bone geometry are independent determinants of bone strength. It has been reported that bone can, and does, adapt its geometry in ways that may not be apparent in bone mineral density (BMD) outcomes (21). To our knowledge, there are no published studies on adipokines and bone geometry, and thus, significant knowledge gaps remain in this area. Third, despite well-observed gender differences in body composition and bone development during adolescence (22), very few studies examined gender-specific association of adipokines with bone phenotypes, which may shed light on gender differences in osteoporosis in later life.
In this study, we examined the associations of plasma adiponectin and leptin with an array of bone phenotypes [bone area (BA), bone mass, and hip geometry] in a large sample of Chinese adolescents. We were particularly interested in whether there is a gender difference in adipokine-bone association, and whether the adipokine-bone association is modified by mechanical loading and fat mass (FM). Using our unique twin design, we also estimated the degree to which shared genetic factors can contribute to the observed adipokine-bone associations.
Subjects and Methods
Study population
This study is part of an ongoing community-based twin study with the goal to identify precursors of metabolic syndrome and osteoporosis. Detailed information on population enrollment at the baseline and follow-up study was published previously (23). Briefly, the study population was recruited in 1998–2000 in the rural area of Anqing City, China (the baseline study). Twins who participated in the baseline study have been followed from 2005 to the present. Written informed consent was obtained from each participant. This study was approved by the institutional review board of Children’s Memorial Hospital and the Ethics Committee of Anhui Medical University. The study was based on the follow-up data and consisted of 1278 adolescents aged 13–21 yr who have completed a questionnaire interview, anthropometry, dual-energy x-ray absorptiometry (DEXA) scan, and plasma adipokine measurements.
Questionnaire
A comprehensive questionnaire was used to collect the participants’ demographic, disease history, and lifestyle information. Physical activity was assessed with the short version of the international physical activity questionnaire (IPAQ-short) (http://www.ipaq.ki.se/ipaq.htm), which has been validated in multiple countries (24,25,26), including China (25,26). Detailed information on physical activity has been described elsewhere (23) and was included in supplemental data published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.
Anthropometry
Height was measured without shoes to the nearest 0.1 cm on a portable calibrated stadiometer. Weight was measured without shoes to the nearest 0.1 kg with the subject standing motionless in the center of a calibrated scale. Tanner stage (I to V) was determined based on visual inspection by a physician (27,28).
DEXA scan
DEXA (GE-Lunar Prodigy, Waukesha, WI; software, enCORE 6.0) was used to measure soft-tissue body composition, bone mineral content (BMC), and BA through whole-body, lumbar spine, and total-hip scans. Whole-body FM and lean mass (LM) were expressed in terms of weight (kilograms). With the manufacturer’s hip structure analysis program, hip geometry variables were automatically calculated from the scanned image and bone distribution variables derived from information contained within DEXA x-ray absorption curves (29). In this study, two variables were studied: 1) cross-sectional area (CSA) of the minimum cross-sectional moment of inertia section within the femoral neck region of interest; and 2) section modulus (SM), which was calculated as the minimum cross-sectional moment of inertia within the femoral neck region of interest divided by distance from the center of mass to the superior neck margin for the section of minimum CSA.
Adipokine measurement
Plasma leptin and plasma total adiponectin were measured by a sandwich immunoassay based on flow metric xMAP technology on Luminex 200 (Luminex Corp., Austin, TX), as described previously (30). In this study, the assay sensitivity for leptin and adiponectin was 138.8 and 145.4 pg/ml, respectively, and the intraassay coefficient of variation for leptin and adiponectin was 5.1 and 8.0%, respectively.
Zygosity identification
Twin zygosity was determined using DNA fingerprint technology by genotyping 10 microsatellite markers on different somatic chromosomes with high heterozygosity (>70%), as described previously (31).
Statistical analysis
The primary outcomes were whole-body less head BA (WB-BA) and BMC (WB-BMC), lumbar spine BA (L2L4-BA) and BMC (L2L4-BMC), total-hip BA (TH-BA) and BMC (TH-BMC), CSA and SM, as well as their corresponding Z-scores. BMD at the three skeletal sites, although reported as an inappropriate marker for assessing bone status in growing children (32), was also analyzed. The independent variables, leptin and adiponectin, were log-transformed to achieve normal distribution. For each bone phenotype and log-transformed adipokine, the values with at least 4 sd values away from the respective average were considered as outliers and were removed from the analyses (n = 28).
Analyses of each BMC were adjusted for the corresponding BA by including it in the linear regression model. All the analyses were conducted in males and females separately. First, bone phenotypes were regressed onto the log-transformed leptin or adiponectin, separately. The basic model was adjusted for age, Tanner stage, height, menarche status (no menarche, menarche at ≤13 yr, menarche at 14 yr, menarche at ≥15 yr), physical activity, student/nonstudent, and corresponding BA (for BMC only). Second, to evaluate the effect of mechanical loading on the adipokine-bone relationships, we compared the following three different regression models by adding the three covariates [LM, body weight, and body mass index (BMI)] separately and sequentially into the basic model. Furthermore, we ran the regression models by adding LM and FM simultaneously into the basic model to investigate whether the adipokine-bone relationships were independent of FM. In each regression model, collinearity was tested by taking 1) variance inflation factor greater than 10; and/or 2) condition indices greater than 15 as an indication for possible problems with collinearity. Any regression models having potential problems with collinearity will be removed. These analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC).
The genetic/environmental correlations between adipokine and bone phenotypes were derived from bivariate Cholesky decomposition models using Mx software (33). We first applied structural equation modeling to divide the phenotypic variance of each trait into the following components: an additive genetic component (a2), shared (c2) and individual-specific (e2) environmental components. Based on different combinations of these components, three different models were developed, including ACE (the full model), AE (set c2 = 0), and CE (set a2 = 0) models. The best-fitting model was defined as the one with the lowest Akaike Information Criteria and not having a significant worse fit when compared with the ACE model. We then fitted the best Bivariate Cholesky decomposition models to calculate genetic (rG) and individual-specific environmental correlations (rE) for each adipokine-bone trait pair using the same criteria. The genetic contribution (CGCP) and individual-specific environmental contribution (CECP) to the phenotypic correlations could be estimated as 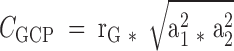 and
and 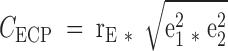 , respectively. In genetic analyses, adjustment for important covariates can generally increase the genetic signal-to-noise ratio by decreasing the proportion of the residual phenotypic variation attributable to random environmental factors. In our analyses, adipokines and bone phenotypes were regressed onto body weight plus the covariates included in the above basic model, and the residuals from the models were used in the analyses to estimate genetic contributions to the above traits and their correlations.
, respectively. In genetic analyses, adjustment for important covariates can generally increase the genetic signal-to-noise ratio by decreasing the proportion of the residual phenotypic variation attributable to random environmental factors. In our analyses, adipokines and bone phenotypes were regressed onto body weight plus the covariates included in the above basic model, and the residuals from the models were used in the analyses to estimate genetic contributions to the above traits and their correlations.
Results
The study population consisted of 675 males and 575 females with the mean ages of 16.5 ± 2.0 yr and 16.7 ± 2.1 yr, respectively. A little over 97% of the subjects were successfully measured for plasma adiponectin and leptin, respectively. Compared with females, males had lower BMI and FM, as well as lower log-transformed plasma adiponectin and leptin (all P < 0.0001), but males had higher values in body weight, height, LM, BA, and BMC at the three sites, CSA, and SM (all P < 0.0001) (Table 1).
Table 1.
Demographic characteristics of 1250 Chinese adolescents aged 13–21 yr
| Variables | Males | Females | Pa |
|---|---|---|---|
| n | 675 | 575 | |
| Age (yr) | 16.5 ± 2.0 | 16.7 ± 2.1 | 0.197 |
| Weight (kg) | 48.6 ± 8.1 | 46.3 ± 6.7 | <0.0001 |
| Height (cm) | 160.7 ± 7.8 | 152.5 ± 5.3 | <0.0001 |
| BMI (kg/m2) | 18.7 ± 2.2 | 19.9 ± 2.5 | <0.0001 |
| BMI Z-scoreb | −0.04 ± 0.98 | −0.04 ± 1.01 | 0.476 |
| Body composition (kg) | |||
| FM | 5.8 ± 3.5 | 12.9 ± 4.3 | <0.0001 |
| LM | 41.3 ± 6.5 | 31.6 ± 3.2 | <0.0001 |
| Adiponectin (μg/ml) | 19.0 ± 10.9 | 22.4 ± 12.5 | <0.0001 |
| ln(Adiponectin) | 2.8 ± 0.6 | 2.9 ± 0.6 | <0.0001 |
| Leptin (pg/ml) | 2059.7 ± 2421.1 | 8388.9 ± 5551.6 | <0.0001 |
| ln(Leptin) | 7.3 ± 0.7 | 8.8 ± 0.7 | <0.0001 |
| BMC (g) | |||
| WB | 1498.9 ± 350.2 | 1324.2 ± 233.6 | <0.0001 |
| Lumbar spine | 36.8 ± 9.8 | 35.6 ± 6.8 | <0.0001 |
| TH | 28.3 ± 5.7 | 23.9 ± 3.5 | <0.0001 |
| BA (cm2) | |||
| WB | 1658.3 ± 244.1 | 1521.1 ± 181.2 | <0.0001 |
| Lumbar spine | 38.9 ± 5.7 | 35.8 ± 3.9 | <0.0001 |
| TH | 31.0 ± 3.3 | 27.0 ± 2.0 | <0.0001 |
| Hip geometric indices | |||
| CSA (cm2) | 1.36 ± 0.25 | 1.17 ± 0.16 | <0.0001 |
| SM (cm3) | 0.55 ± 0.14 | 0.42 ± 0.08 | <0.0001 |
| Tanner stage | |||
| I | 60 (8.9) | 8 (1.4) | |
| II | 110 (16.3) | 91 (15.8) | |
| III | 132 (19.5) | 215 (37.4) | |
| IV | 143 (21.2) | 149 (25.9) | |
| V | 133 (19.7) | 104 (18.1) | |
| Unknown | 97 (14.4) | 8 (1.4) | <0.0001 |
| Menarche status | |||
| No | 66 (11.5) | ||
| Menarche at ≤13 yr | 214 (37.3) | ||
| Menarche at 14 yr | 150 (26.1) | ||
| Menarche at ≥15 yr | 145 (25.2) | ||
| Physical activity | |||
| Low | 209 (31.0) | 226 (39.3) | |
| Moderate | 237 (35.1) | 192 (33.4) | |
| Heavy | 158 (23.4) | 95 (16.5) | |
| Unknown | 71 (10.5) | 62 (10.8) | 0.003 |
| Student (yes) | 502 (74.4) | 374 (65.0) | 0.0003 |
| Passive smoking (yes) | 476 (72.8) | 374 (68.6) | 0.105 |
| Current smoking (yes) | 64 (9.5) | 0 | <0.0001 |
Mean ± sd is shown for continuous variables, and number (percentage) is shown for categorical variables.
t test and χ2 test were performed to compare gender difference in continuous variables and categorical variables, respectively.
Using the subset of our samples, which was composed of unrelated subjects drawn randomly from each twin pair, as the reference group.
Age of menarche, plasma adipokines, and bone phenotypes
A total of 509 (88.5%) females were postmenarche. The average age of menarche was 13.8 ± 1.3 yr. We plotted log-transformed adipokine Z-scores and bone phenotype Z-scores against age of menarche (Supplemental Fig. 1). We found that plasma leptin, WB-BA, and WB-BMC decreased significantly with age of menarche. Similar patterns were found for L2L4-BA,TH-BA, L2L4-BMC, and TH-BMC (data not shown). CSA decreased with increasing age of menarche in nonlinear fashion. In comparison, no significant relationship was found for plasma adiponectin or for SM.
Relationship between plasma adipokines and bone phenotypes
Table 2 illustrates the adiponectin-bone relationships, with and without adjustment for mechanical loading and/or FM. In males, adiponectin was negatively associated with WB-BMC (β ± se = −21.46 ± 7.25; P = 0.003), L2L4-BMC (β ± se = −1.13 ± 0.30; P = 0.0001), and TH-BMC (β ± se = −1.12 ± 0.27; P < 0.0001) in the basic models, which were not affected by adjustment for LM, body weight, or BMI singly, or for LM and FM simultaneously. Adiponectin was negatively associated with WB-BA, CSA, and SM (P = 0.002–0.044) in the basic model, but these associations were attenuated and became insignificant after adjusting for any of the above-mentioned covariates. In females, we only observed a marginal but positive association between adiponectin and TH-BA (P < 0.05) (Table 2).
Table 2.
Association of log-transformed adiponectin with bone phenotypes in 1250 Chinese adolescents aged 13–21 yr, with adjustment of different body compositions
| Regression models | WB-BA | L2L4-BA | TH-BA | WB-BMC | L2L4-BMC | TH-BMC | CSA | SM |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| Basic modela | −25.23 ± 8.31c | 0.02 ± 0.22 | 0.05 ± 0.14 | −21.46 ± 7.25c | −1.13 ± 0.30d | −1.12 ± 0.27e | −0.04 ± 0.01c | −0.01 ± 0.01b |
| Basic model+LM | −13.87 ± 6.38b | 0.20 ± 0.21 | 0.20 ± 0.13 | −21.53 ± 7.24c | −0.99 ± 0.29d | −0.88 ± 0.25d | −0.02 ± 0.01 | −0.01 ± 0.01 |
| Basic model+BW | −9.73 ± 6.39 | 0.19 ± 0.22 | 0.18 ± 0.15 | −20.73 ± 7.18c | −0.94 ± 0.29c | −0.88 ± 0.25d | −0.02 ± 0.01 | −0.01 ± 0.01 |
| Basic model+BMI | −10.08 ± 6.47 | 0.19 ± 0.22 | 0.18 ± 0.15 | −20.96 ± 7.20c | −0.95 ± 0.29c | −0.89 ± 0.25d | −0.02 ± 0.01 | −0.01 ± 0.01 |
| Basic model+LM+FM | −9.62 ± 5.86 | 0.19 ± 0.21 | 0.19 ± 0.13 | −21.10 ± 7.21c | −0.94 ± 0.29c | −0.84 ± 0.24d | −0.02 ± 0.01 | −0.01 ± 0.01 |
| Females | ||||||||
| Basic modela | −8.04 ± 8.65 | 0.02 ± 0.21 | 0.26 ± 0.13b | −6.45 ± 5.36 | −0.56 ± 0.28b | −0.10 ± 0.21 | −0.01 ± 0.01 | 0.00 ± 0.01 |
| Basic model+LM | −6.15 ± 7.16 | 0.04 ± 0.20 | 0.28 ± 0.12b | −6.58 ± 5.30 | −0.54 ± 0.23 | 0.00 ± 0.19 | −0.01 ± 0.01 | 0.00 ± 0.01 |
| Basic model+BW | −1.27 ± 5.51 | 0.08 ± 0.20 | 0.31 ± 0.12b | −6.31 ± 5.34 | −0.49 ± 0.27 | 0.05 ± 0.19 | −0.01 ± 0.01 | 0.00 ± 0.01 |
| Basic model+BMI | −1.03 ± 5.61 | 0.08 ± 0.20 | 0.31 ± 0.12b | −6.32 ± 5.34 | −0.48 ± 0.27 | 0.06 ± 0.19 | −0.01 ± 0.01 | 0.00 ± 0.01 |
| Basic model+LM+FM | 1.11 ± 5.60 | 0.09 ± 0.20 | 0.30 ± 0.12b | −7.04 ± 5.35 | −0.48 ± 0.27 | 0.06 ± 0.19 | −0.01 ± 0.01 | 0.00 ± 0.01 |
Data are expressed as β (parameter estimate) ± se. LM, Whole-body LM; FM, whole-body FM; BW, body weight.
Basic model adjusted for age, Tanner stage, height, menarche status (no menarche, menarche at ≤13 yr, menarche at 14 yr, menarche at ≥15 yr), physical activity, active or passive smoking (yes/no), student/nonstudent, and corresponding BA (for BMC only).
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
The leptin-bone relationship was shown in Table 3. In males, no significant relationships, except for WB-BA, were observed in the basic model or in the LM-adjusted model. After adding body weight to the basic model, leptin was inversely associated with WB-BA (β ± se = −25.71 ± 5.19; P < 0.0001), L2L4-BA (β ± se = −0.84 ± 0.17; P < 0.0001), TH-BA (β ± se = −0.69 ± 0.13; P < 0.0001), L2L4-BMC (β ± se = −0.61 ± 0.27; P = 0.024), TH-BMC (β ± se = −0.65 ± 0.22; P = 0.003), CSA (β ± se = −0.05 ± 0.01; P < 0.0001), and SM (β ± se = −0.03 ± 0.01; P = 0.0002). Similar results were observed in the BMI-adjusted model. When LM and FM were added to the basic model instead of body weight, the leptin-bone associations were no longer significant. Similar patterns for TH-BMC, CSA, and SM were observed in females. However, the negative associations of leptin with WB-BA and L2L4-BA in females remained significant (P < 0.05) even after adjustment for both LM and FM. Furthermore, the corresponding age- and gender-specific quartiles of leptin, body weight, and BMI were calculated and then were applied to test the leptin × body weight interaction and the leptin × BMI interaction separately, after adjusting for the covariates in the basic model. We only found a marginal leptin × BMI interaction on TH-BMC (P = 0.04) in males and on WB-BA (P = 0.02) in females (data not shown).
Table 3.
Association of log-transformed leptin with bone phenotypes in 1250 Chinese adolescents aged 13–21 yr, with adjustment of different body compositions
| Regression models | WB-BA | L2L4-BA | TH-BA | WB-BMC | L2L4-BMC | TH-BMC | CSA | SM |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| Basic modela | 25.22 ± 6.45d | −0.16 ± 0.15 | −0.16 ± 0.11 | 2.82 ± 5.36 | 0.13 ± 0.23 | 0.28 ± 0.20 | 0.01 ± 0.01 | 0.00 ± 0.01 |
| Basic model+LM | 23.33 ± 4.83e | −0.19 ± 0.15 | −0.19 ± 0.10 | 4.44 ± 5.23 | 0.09 ± 0.23 | 0.18 ± 0.18 | 0.00 ± 0.01 | 0.00 ± 0.01 |
| Basic model+BW | −25.71 ± 5.19e | −0.84 ± 0.17e | −0.69 ± 0.13e | −3.98 ± 6.28 | −0.61 ± 0.27b | −0.65 ± 0.22c | −0.05 ± 0.01e | −0.03 ± 0.01d |
| Basic model+BMI | −25.68 ± 5.31e | −0.84 ± 0.17e | −0.70 ± 0.13e | −1.95 ± 6.27 | −0.58 ± 0.27b | −0.61 ± 0.22c | −0.05 ± 0.01e | −0.03 ± 0.01d |
| Basic model+LM+FM | −4.18 ± 5.36 | −0.21 ± 0.18 | −0.17 ± 0.12 | 0.77 ± 6.35 | −0.43 ± 0.27 | −0.09 ± 0.23 | −0.01 ± 0.01 | −0.01 ± 0.01 |
| Females | ||||||||
| Basic modela | 52.02 ± 8.28e | 0.08 ± 0.09 | 0.21 ± 0.12 | −5.35 ± 5.88 | 0.51 ± 0.25c | 0.43 ± 0.22b | 0.02 ± 0.01b | 0.01 ± 0.01 |
| Basic model+LM | 32.96 ± 7.05e | −0.16 ± 0.19 | −0.01 ± 0.11 | −6.57 ± 5.86 | 0.28 ± 0.25 | 0.07 ± 0.21 | 0.00 ± 0.01 | 0.00 ± 0.00 |
| Basic model+BW | −20.50 ± 5.67d | −0.55 ± 0.21b | −0.33 ± 0.12c | −8.44 ± 6.32 | −0.24 ± 0.29 | −0.60 ± 0.23c | −0.03 ± 0.01c | −0.01 ± 0.01c |
| Basic model+BMI | −21.71 ± 5.73d | −0.58 ± 0.21c | −0.36 ± 0.12c | −8.69 ± 6.35 | −0.29 ± 0.29 | −0.64 ± 0.23c | −0.03 ± 0.01c | −0.01 ± 0.01c |
| Basic model+LM+FM | −20.96 ± 5.88d | −0.51 ± 0.22b | −0.18 ± 0.13 | −0.81 ± 6.81 | −0.14 ± 0.30 | −0.30 ± 0.24 | −0.02 ± 0.01 | −0.01 ± 0.01 |
Data are expressed as β (parameter estimate) ± se. LM, Whole-body LM; FM, whole-body FM; BW, body weight.
Basic model adjusted for age, Tanner stage, height, menarche status (no menarche, menarche at ≤13 yr, menarche at 14 yr, menarche at ≥15 yr), physical activity, active or passive smoking (yes/no), student/nonstudent, and corresponding BA (for BMC only).
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
The adipokine-BMD relationships at the three skeletal sites were also analyzed in both genders (Supplemental Table 1), which showed that their relationships were comparable with those between adipokine and BMC. We further repeated the above analyses by using the corresponding Z-scores of each bone phenotype and of each log-transformed adipokine as the outcome and predictor, respectively. Continuous covariates in the regression models, including height, were also transferred into the corresponding Z-score values. We found that our results on the adipokine-bone relationship remained unchanged (Supplemental Tables 2–4).
Genetic/environmental contribution to the adipokine-bone relationship
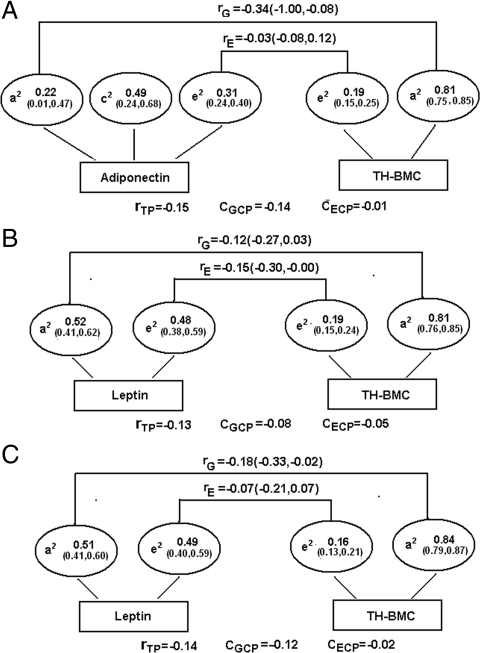
We then estimated the degree to which genetics can contribute to the observed adipokine-bone associations. Based on results from the multiple regression analyses, we limited these genetic analyses to the adiponectin–TH-BMC pair in males and the leptin–TH-BMC pair in both genders. These analyses were conducted in a subset of subjects with available zygosity information, including 336 monozygotic twins (males, 157 pairs; females, 179 pairs) and 181 dizygotic twins (males, 103 pairs; females, 78 pairs).
The bivariate structural equation model for each pair of traits was fitted based on the best-fitting univariate model of each trait, which was the ACE model for adiponectin and the AE model for leptin and TH-BMC, respectively (Supplemental Table 5). The genetic correlations (rG) for the adiponectin–TH-BMC pair in males and for the leptin–TH-BMC pair in males and females were −0.34 [95% confidence interval (CI), −1.00, −0.08], −0.12 (95% CI, −0.27, 0.03), and −0.18 (95% CI, −0.33, −0.02), respectively, suggesting a significant or marginally significant genetic contribution to the adipokine–TH-BMC associations (Fig. 1). Genetic factors contributed about 93% (= −0.14/−0.15), 62% (= −0.08/−0.13), and 0.86% (= −0.12/−0.14) to the total phenotypic correlation between adiponectin and TH-BMC in males and between leptin and TH-BMC in males and females, respectively.
Figure 1.
The genetic and individual-specific environmental contribution to the adiponectin–TH-BMC association (A) and to the leptin–TH-BMC association (B) in 260 male adolescent twin pairs, as well as to the leptin–TH-BMC association in 257 female adolescent twin pairs (C). a2, c2, and e2 denote percentage of total phenotypic variance accounted for by genetic factors, shared environmental factors, and individual-specific environmental factors, respectively; rG and rE, genetic and individual-specific environmental correlation, respectively; rTP, total phenotypic correlation; CGCP, genetic contribution to total phenotypic correlation; CECP, individual-specific contribution to total phenotypic correlation.
Discussion
To our knowledge, this study was one of the largest studies to investigate the relationship between adipokines and an array of bone phenotypes in Chinese adolescents. We demonstrated that adiponectin was inversely and independently associated with BMC (or BMD) at the three skeletal sites in males but not in females. No independent relationships were detected between adiponectin and hip geometry in both genders. In comparison, the negative associations between leptin and multiple bone phenotypes were greatly dependent on the adjustment for mechanical loading (body weight or BMI) in both genders, which disappeared when FM and LM were simultaneously adjusted. We also demonstrated that, to a large degree, the phenotypic correlations between adiponectin and TH-BMC in males and between leptin and TH-BMC in both genders were attributed to shared genetic factors.
The effect of adiponectin on adolescent bone health remains largely unexplored. To date, only two small studies (n < 110) have been conducted, and both were in females only (13,14). The study by Huang et al. (14) reported no association in healthy, nondiabetic Chinese girls after adjusting for age, BMI (or FM), and Tanner stage in their model. Misra et al. (13) observed negative associations between adiponectin and multiple bone parameters in 17 American girls with anorexia nervosa and 19 healthy controls after adjustment of BMI, lean mass, insulin, leptin, IGF-I, GH, ghrelin, estradiol, cortisol, and peptide YY. In comparison, we found no significant associations between adiponectin and all the BMC measures in females, confirming the observations by Huang et al. (14).
This is the first study to examine the association of adiponectin and bone phenotypes in adolescent males. Our findings on BMCs are consistent with the negative associations found in adult men (34,35). Our study further indicated that such negative association in males appeared to mainly affect bone material rather than BA. Although the underlying mechanisms remain unknown, we speculate that adiponectin can inversely affect bone mass by stimulating RANKL and inhibiting osteoprotegerin production in osteoblast (8). Another explanation is that adiponectin, which is negatively associated with bioavailable estradiol concentrations in both genders (36), may affect bone mass indirectly by decreasing estrogen levels. The latter pathway, if proved to be true, may partly explain why the negative effect of adiponectin on bone mass is insignificant in females, because most of our female subjects were postmenarche and, as such, may have sufficient circulating estrogen. It is likely that females with a relatively higher level of adiponectin still have enough circulating estrogen to stimulate bone growth, and thus, the negative effect of adiponectin on bone can be alleviated. Surprisingly, we found a modest but positive association between adiponectin and TH-BA in females, indicating that actions of adiponectin may vary by bone phenotypes. A previous study has reported that circulating adiponectin has both the direct/negative and indirect/positive action on bone formation and that the indirect/positive action may be mediated via enhancement of the insulin signaling (37). However, further studies are needed to determine whether the positive association we found in females is mediated by the enhanced insulin signaling.
Previous studies in children or adolescents have yielded mixed results between leptin and BMC or BMD (13,14,15,16,17,18). These inconsistencies may be due in part to different selection criteria of the study subjects (i.e. difference in ethnicity, age, gender, etc.), limited statistical power in some studies (13,14,15,16,17,18), and/or the varying approaches to statistically adjusting for the confounding effects of mechanical load and/or FM. Our study supported a finding that the magnitude and direction of the associations between leptin and bone phenotypes are greatly dependent on the adjustment for mechanical loading and/or FM in statistical models. Body weight or BMI is often used as a surrogate for mechanical load on bone, and it is widely accepted that a high body weight or BMI leads to high bone strength. Body weight or BMI is also strongly associated with metabolic parameters such as adipokines. Failure to adjust for body weight or BMI may confound the associations between adipokines and bone phenotypes. Our data demonstrated that leptin was negatively associated with all BAs, TH-BMC, WB-BMD, and TH-BMD in both genders when adjusting for body weight or BMI. Notably, adjusting for body weight or BMI does not directly take into account body composition (FM vs. LM), and therefore, it may not account for the nonmechanical effects of FM itself on bone, which has been shown to be negative (2,3). We further adjusted LM and FM simultaneously, instead of body weight, and found that the negative associations between leptin and bone phenotypes (except for WB-BA and L2L4-BA in females) were no longer significant. Taken together, our findings suggest that the negative associations between leptin and bone phenotypes can be explained by FM. This is consistent with previous reports suggesting that leptin may be one of many adipokines that mediate the hormonal effects of adipose tissue on bone mass (38).
Although hip geometry has received increasing attention recently, there are no reports about the relationship between adipokines and hip geometry in adolescents. A strength of this study is that we have compared the effect of adipokines on bone mass and on two main hip geometric variables (CSA and SM). We demonstrated that, although adiponectin was inversely and independently associated with BMC (or BMD) in males, the association between adiponectin and hip geometry disappeared after adjusting for body weight. These findings indicate that the action of adiponectin on bone varies by bone material vs. geometry. In contrast, leptin appears to affect both bone mass and hip geometry in our study.
Our study also demonstrated that the observed inverse associations between adiponectin and TH-BMC in males and between leptin and TH-BMC in both genders were contributed by genetic factors. This finding is consistent with our current understanding about the reciprocal differentiation of adipocyte (the main origin of adipokines) and osteoblasts, both originating from the same mesenchymal stem cells. Mesenchymal stem cells can differentiate into either osteoblasts or adipocytes in a mutually exclusive manner, which is regulated by two key transcription factors, Runx2 and peroxisome proliferator- activated receptor γ. Thus, any genetic factors influencing the expression of Runx2 and peroxisome proliferator-activated receptor γ may contribute to the inverse correlation between adipokine and bone health. It would be interesting to conduct bivariate linkage studies or bivariate genome-wide association studies to identify the loci or genes underlying adipokine-bone phenotypic correlations in adolescents.
We acknowledge that our study has some limitations, and our findings should be interpreted within its context. First, our study was limited to cross-sectional analysis and thus could not elucidate the temporal relationship between adipokines and bone health. Second, hip geometry variables in this study, which were estimated through DEXA measurement, were subject to certain technical limitations (39). Third, data on bone age, gonadal steroid, and dietary information (such as vitamin D and calcium intake) were currently not available in this study. Further studies are needed to determine whether the associations between adipokines and bone phenotypes could be confounded by these factors. Fourth, the IPAQ-short questionnaire applied in this study, which was designed and validated for subjects aged 15 yr or older, may not estimate physical activity level accurately in adolescents aged less than 15 yr. Also, our data showed that 88.5% of the females have attained menarche, but only 44% reported as having Tanner IV or V pubertal status, which may suggest that there was an underweighting of the Tanner stage in females. However, these limitations may not have significant influence on our findings because we found that the relationships between adipokine and bone phenotypes remained unchanged with or without adjustment of physical activity and/or Tanner stage. Finally, although the twin design allows for the estimation of the genetic correlation between two phenotypes, one should be cautious to generalize our findings to non-twin populations. Nevertheless, our previous reports showed that this twin cohort was similar to the local population with regard to socioeconomic characteristics, lifestyles, and anthropometric measurements (40).
In conclusion, in this sample of relatively lean, healthy Chinese adolescents, we demonstrated that adiponectin and leptin were inversely associated with bone phenotypes, but appeared to have differential associations by gender, bone material vs. geometry parameters, and adjustment of FM. Our data underscore the importance of genetic influence on the phenotypic correlations between adipokines and bone phenotypes in both genders. Further studies are needed to identify specific genes or gene-environmental interactions underlying the phenotypic correlations.
Supplementary Material
Acknowledgments
We acknowledge the assistance and cooperation of the faculty and staff of the Anhui Institute of Biomedicine, Anhui Medical University, and thank all study participants for their support.
Footnotes
This work was supported by Grants R01 HD049059 from the National Institute of Child Health and Human Development, R01 HL0864619 from the National Heart, Lung, and Blood Institute, R01 AG032227 from the National Institute of Aging, and 30700880 from the Chinese Science Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 10, 2010
Abbreviations: BA, Bone area; BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; CSA, cross-sectional area; DEXA, dual-energy x-ray absorptiometry; FM, fat mass; L2L4-BA, lumbar spine BA; LM, lean mass; RANKL, receptor activator of nuclear factor-κB ligand; SM, section modulus; TH, total hip; WB, whole-body less head.
References
- Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW 2007 Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V 2007 Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab 92:143–147 [DOI] [PubMed] [Google Scholar]
- Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD 2007 Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr 86:1530–1538 [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G 2000 Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- Karsenty G 2006 Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4:341–348 [DOI] [PubMed] [Google Scholar]
- Cirmanova V, Bayer M, Starka L, Zajickova K 2008 The effect of leptin on bone: an evolving concept of action. Physiol Res 57(Suppl 1):S143–S151 [DOI] [PubMed] [Google Scholar]
- Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE 2004 Adiponectin and its receptors are expressed in bone-forming cells. Bone 35:842–849 [DOI] [PubMed] [Google Scholar]
- Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY 2006 Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res 21:1648–1656 [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC 2001 Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab 86:1884–1887 [DOI] [PubMed] [Google Scholar]
- Tamura T, Yoneda M, Yamane K, Nakanishi S, Nakashima R, Okubo M, Kohno N 2007 Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism 56:623–628 [DOI] [PubMed] [Google Scholar]
- Kontogianni MD, Dafni UG, Routsias JG, Skopouli FN 2004 Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res 19:546–551 [DOI] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Burling K, Perks UC, Spector TD 2007 Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab 92:1517–1523 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, Katzman DK, Klibanski A 2007 Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab 92:2046–2052 [DOI] [PubMed] [Google Scholar]
- Huang KC, Cheng WC, Yen RF, Tsai KS, Tai TY, Yang WS 2004 Lack of independent relationship between plasma adiponectin, leptin levels and bone density in nondiabetic female adolescents. Clin Endocrinol (Oxf) 61:204–208 [DOI] [PubMed] [Google Scholar]
- Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A 2008 Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab 93:1292–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich JN, Clark PA, Mantzoros CS, Gurgol CM, Weltman A, Rogol AD 2003 Relationship of leptin to bone mineralization in children and adolescents. J Clin Endocrinol Metab 88:599–604 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Potau N, Ong K, Dunger DB, De Zegher F 2000 Increased bone mineral density and serum leptin in non-obese girls with precocious pubarche: relation to low birthweight and hyperinsulinism. Horm Res 54:192–197 [DOI] [PubMed] [Google Scholar]
- Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG 1998 Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab 83:3469–3475 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C 2000 Peak bone mass. Osteoporos Int 11:985–1009. [DOI] [PubMed] [Google Scholar]
- 2004 Bone health and osteoporosis: a report of the Surgeon General. Rock-ville, MD: US Department of Health and Human Services [Google Scholar]
- Järvinen TL, Kannus P, Sievänen H 1999 Have the DXA-based exercise studies seriously underestimated the effects of mechanical loading on bone? J Bone Miner Res 14:1634–1635 [DOI] [PubMed] [Google Scholar]
- Ouyang F, Wang B, Arguelles LM, Xu X, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, Langman C, Wang X 2007 Bone growth patterns in Chinese children and adolescents: a 6-year follow-up study provides evidence for sexual dimorphism and tracking. Arch Osteoporos 2:29–43 [Google Scholar]
- Yu Y, Lu BS, Wang B, Wang H, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, Xu X, Zee PC, Wang X 2007 Short sleep duration and adiposity in Chinese adolescents. Sleep 30:1688–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P 2003 International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395 [DOI] [PubMed] [Google Scholar]
- Macfarlane DJ, Lee CC, Ho EY, Chan KL, Chan DT 2007 Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport 10:45–51 [DOI] [PubMed] [Google Scholar]
- Deng HB, Macfarlane DJ, Thomas GN, Lao XQ, Jiang CQ, Cheng KK, Lam TH 2008 Reliability and validity of the IPAQ-Chinese: the Guangzhou Biobank Cohort study. Med Sci Sports Exerc 40:303–307 [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1969 Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1970 Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Turner CH, Peacock M, Slemenda CW, Weaver CM, Teegarden D, Markwardt P, Burr DB 1994 Geometric structure of the femoral neck measured using dual-energy x-ray absorptiometry. J Bone Miner Res 9:1053–1064 [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Thorsen P, Nørgaard-Pedersen B, Schendel DE, Sørensen LC, Hougaard DM 2005 Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem 51:1854–1866 [DOI] [PubMed] [Google Scholar]
- Wang B, Necheles J, Ouyang F, Ma W, Li Z, Liu X, Yang J, Xing H, Xu X, Wang X 2007 Monozygotic co-twin analyses of body composition measurements and serum lipids. Prev Med 45:358–365 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2003 Bone mineral content, not bone mineral density, is the correct bone measure for growth studies. Am J Clin Nutr 78:350–351; author reply 351–352 [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR 1992 Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY 2008 Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta 387:31–35 [DOI] [PubMed] [Google Scholar]
- Michaëlsson K, Lind L, Frystyk J, Flyvbjerg A, Gedeborg R, Berne C, Zethelius B, Mallmin H, Söderberg S, Melhus H 2008 Serum adiponectin in elderly men does not correlate with fracture risk. J Clin Endocrinol Metab 93:4041–4047 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, May S 2007 Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes (Lond) 31:457–465 [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, Ikeda T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H 2006 Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem 99:196–208 [DOI] [PubMed] [Google Scholar]
- Thomas T, Burguera B 2002 Is leptin the link between fat and bone mass? J Bone Miner Res 17:1563–1569 [DOI] [PubMed] [Google Scholar]
- Beck T 2003 Measuring the structural strength of bones with dual-energy x-ray absorptiometry: principles, technical limitations, and future possibilities. Osteoporos Int 14(Suppl 5):S81–S88 [DOI] [PubMed] [Google Scholar]
- Yu Y, Kumar R, Venners S, Pongracic J, Wang B, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, Xu X, Wang X 2007 Age and gender specific lung function predictive equations provide similar predictions for both a twin population and a general population from age 6 through adolescence. Pediatr Pulmonol 42:631–639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.