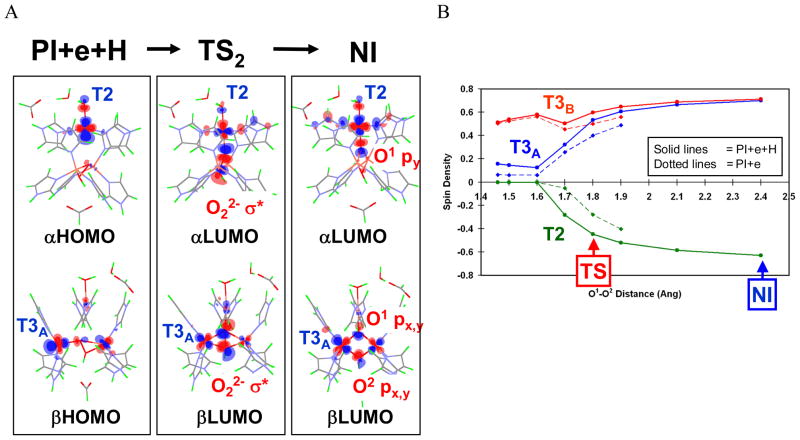
Figure 26.
(A) Molecular orbitals involved in electron transfer during the PI+e+H → TS2 → NI process. In PI+e+H, only the T3B center is oxidized, and the α- and β-HOMOs are derived from the highest-energy d-electrons of T2 and T3A Cu centers. In TS2, both the T2 dx2-y2-based α-HOMO and T3A dx2-y2-based β-HOMO have good overlap and mixing with the peroxide LUMO (O22− σ*) that promotes facile simultaneous two-electron transfer from the donor T2 and T3A Cu’s to the acceptor peroxide for the O-O bond cleavage. Finally, in NI, the T2 and T3A Cu centers are fully oxidized and both O atoms fully reduced to μ3-oxo (O1) and μ-OH (O2) bridging ligands. The same type of MO correlation is present for the PI+e →TS1→ NI process. (B) A plot of the spin density on each of the three Cu’s of the TNC as a function of O-O distance. Solid lines indicate changes from PI+e+H to NI (i.e., with protonated peroxide), and dotted lines indicate changes from PI+e. PI+e with R(O1-O2) > 1.9 Å are not shown, as the proton transfer occurs spontaneously with no barrier at these O1-O2 distances.38