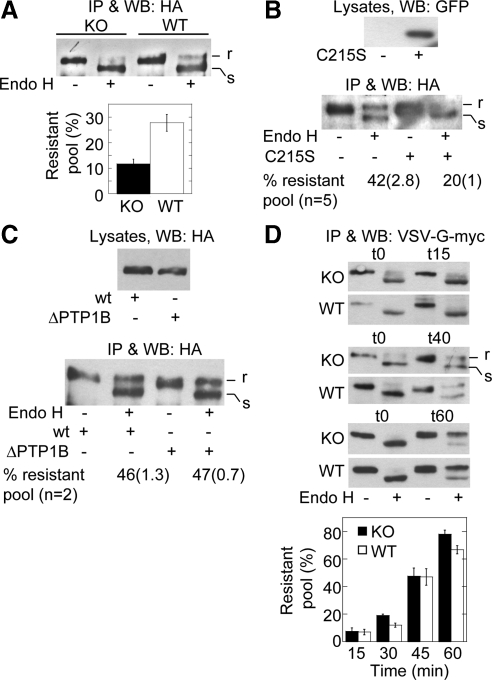
Figure 6.
Expression but not binding of PTP1B to the N-cadherin precursor is selectively required for its passage to the Golgi complex. (A) WT- and KO-cells transfected with HA-N-cadherin-GFP were lysed, and the precursor was isolated by immunoprecipitation with-anti-HA. Immunoprecipitated samples were split in two equal aliquots, and one was digested with endo-H. Proteins were fractionated by SDS-PAGE and immunoblotted with anti-HA. Top, a representative blot is shown indicating endo-H–resistant (r) and –sensitive (s) fractions. Bottom, graph of the quantifications of the endo-H–resistant fractions (expressed as percentages of total). Columns represent means ± SEM (n = 7; Student's t, p = 0.002). (B) WT-cells were cotransfected with HA-N-cadherin-GFP and either empty vector or the dominant negative GFP-PTP1B C215S. Cells were processed for endo-H analysis of the HA-N-cadherin-GFP precursor as before. A representative blot and quantitative data of several experiments are shown. Data represent the mean and SEM in parentheses. Note the reduction of the endo-H–resistant fraction in cells expressing the dominant negative C215S. (C) WT-cells were transfected with HA-N-cadherin-GFP (wt) or HA-N-cadherin-Δ884-891-GFP (ΔPTP1B), a deletion mutant of N-cadherin that cannot bind PTP1B. The precursor was isolated and subjected to endo-H analysis as before. A representative blot of two independent experiments is shown. Note that the precursor of both constructs has similar percentages of endo-H–resistant fractions. (D) WT- and KO-cells were transfected with VSV-G (tsO45)-myc and cultured for 18–20 h at 40°C to accumulate the construct in the ER. Afterward, the temperature was shifted to 32°C to allow trafficking; incubation time (in minutes) is given in parentheses. VSV-G was immunoprecipitated and processed for endo-H treatment as before. The graph below shows the quantification of three to five experiments. Note that endo-H–resistant fractions of VSV-G accumulate in a time-dependent manner and is similar in KO- and WT-cells.