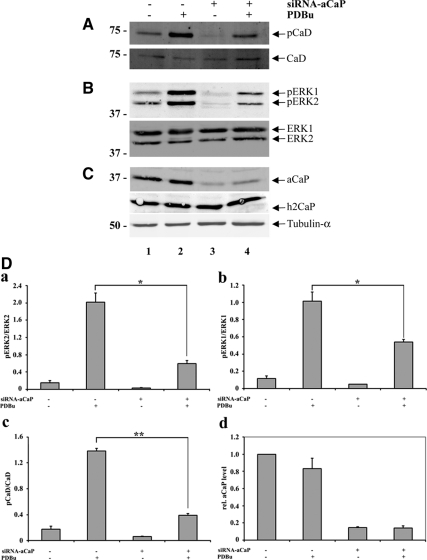
Figure 7.
h3/acidic CaP knockdown inhibits ERK1/2 activation in REF52.2 cells. Seventy-two hours after transfection with siRNA against rat h3/acidic CaP (+, lanes 3 and 4) or with nontargeting control-siRNA (−, lanes 1 and 2), REF52.2 cells were treated with 1 μM PDBu for 30 min (+, lanes 2 and 4) or DMSO as solvent control (−, lanes 1 and 3). Fifty micrograms of whole cell extracts was analyzed by SDS-PAGE and Western blotting with specific antibodies. Arrows indicate the positions of (A) l-caldesmon (CaD), phospho-l-caldesmon (pCaD); (B) ERK1, phospho-ERK1 (pERK1), ERK2, phospho-ERK2 (pERK2); and (C) h3/acidic calponin (aCaP), neutral calponin (h2CaP) and tubulin-α. (D) Densitometric measurement of Western blots, derived from three independent experiments, was performed and total protein levels were normalized to tubulin-α. For phosphorylation levels of ERK1/2 and l-CaD, the ratio of phosphorylated to unphosphorylated protein was calculated and graphed (a–c). The decrease of phosphorylated ERK1 (b; p = 0.0195) and ERK2 (a; p = 0.0478) in h3/acidic CaP knockdown cells stimulated with PDBu was statistically significant, as was the difference of l-CaD phosphorylation (c; p = 0.0012). For relative h3/acidic CaP levels, the amount of h3/acidic CaP in nontargeting control-siRNA transfected and DMSO treated cells was set as 1, and relative h3/acidic CaP levels of the other three samples was calculated (d).