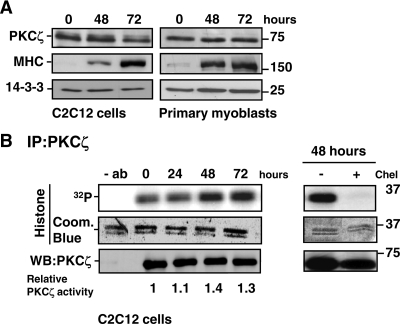
Figure 3.
Expression and activity of PKCζ during myogenesis. (A) The protein levels of PKCζ remained stable during myoblast differentiation. C2C12 cells and primary myoblasts were induced to differentiate, and cells were harvested at the indicated time points. The progress of differentiation was assessed by monitoring the expression of MHC, whereas 14-3-3 served as a loading control. (B) PKCζ activity was up-regulated during myoblast differentiation. C2C12 cells were shifted to differentiation medium, and PKCζ was immunoprecipitated from the cell lysates prepared at the indicated time points. The activity of immunoprecipitated PKCζ was analyzed using histone H1 as a substrate. As a negative control, cell extracts were incubated for 15 min with the PKC inhibitor chelerythrine (Chel, 5 μM) before the activity assay. Coomassie Blue staining was utilized to verify that equal amounts of substrate were used in each reaction, whereas immunoblot analysis confirmed that equal amounts of PKCζ were precipitated from each cell lysate. The blots were scanned on a densitometer to quantitate the amount of PKCζ and the degree of histone 1 phosphorylation. The ratio of 32P-histone 1 and PKCζ at each time point was calculated and normalized to the control values at time point 0 h. The data are representative of three independent experiments.