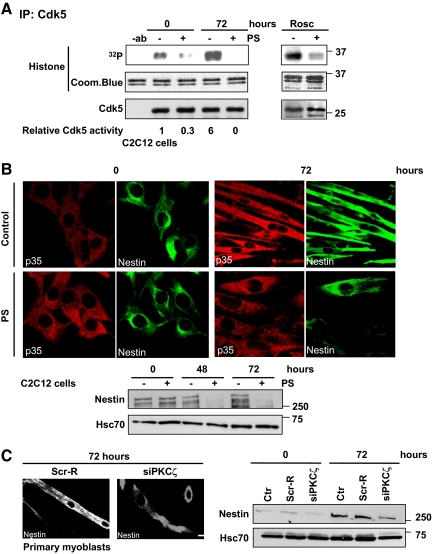
Figure 4.
PKCζ modulates Cdk5 activity in vivo and affects the reorganization of nestin filaments during differentiation. (A) The Cdk5 activity was inhibited upon PKCζ inhibition. C2C12 cells were induced to differentiate for 72 h in the presence or absence of the PKCζ pseudosubstrate inhibitor peptide (PS). Cell lysates were subjected to the immunoprecipitation of endogenous Cdk5 and the Cdk5 kinase activity assays were performed using histone H1 as substrate (left). The numbers represent the relative induction in the Cdk5 activity during differentiation in the presence or absence of the inhibitor peptide PS. The data are representative of three independent experiments. As a negative control, cell lysates were preincubated with the Cdk5 inhibitor roscovitine (Rosc; 5 μM) for 15 min before kinase activity assay (right). Immunoblotting confirmed that equal amounts of Cdk5 were immunoprecipitated from each cell lysate. In addition, Coomassie Blue staining showed that equal amounts of histone 1 substrate were used in each reaction. (B) PKCζ activity was necessary for the reorganization of nestin filaments and the regulation of nestin protein levels during differentiation. C2C12 myoblasts were induced to differentiate for 72 h in the presence or absence of the inhibitor peptide (PS). The samples were fixed and double-stained with antibodies recognizing nestin and p35. At 72 h of differentiation, nestin filaments were aligned parallel to myotubes and colocalized with p35. However the Cdk5-mediated reorganization of nestin filaments during differentiation was disturbed when PKCζ activity was inhibited (scale = 10 μm, as in panel C). In parallel cell lysates were prepared from the same experiment, proteins were resolved by SDS-PAGE and analyzed by Western blotting for nestin and Hsc70, the latter serving as a loading control. The immunoblot revealed pronounced decreases in nestin protein levels when PKCζ was inhibited. (C) The down-regulation of PKCζ severely impaired nestin reorganization and stability during differentiation of primary myoblasts. Mouse primary myoblasts were transfected with PKCζ siRNA (siPKCζ) or scrambled siRNA (Scr-R) and induced to differentiate for 72 h. Samples were fixed, stained with anti-nestin antibody (scale bar, 10 μm), and analyzed by Western blotting. The results confirmed alterations in nestin remodeling and turnover observed during differentiation when PKCζ was down-regulated.